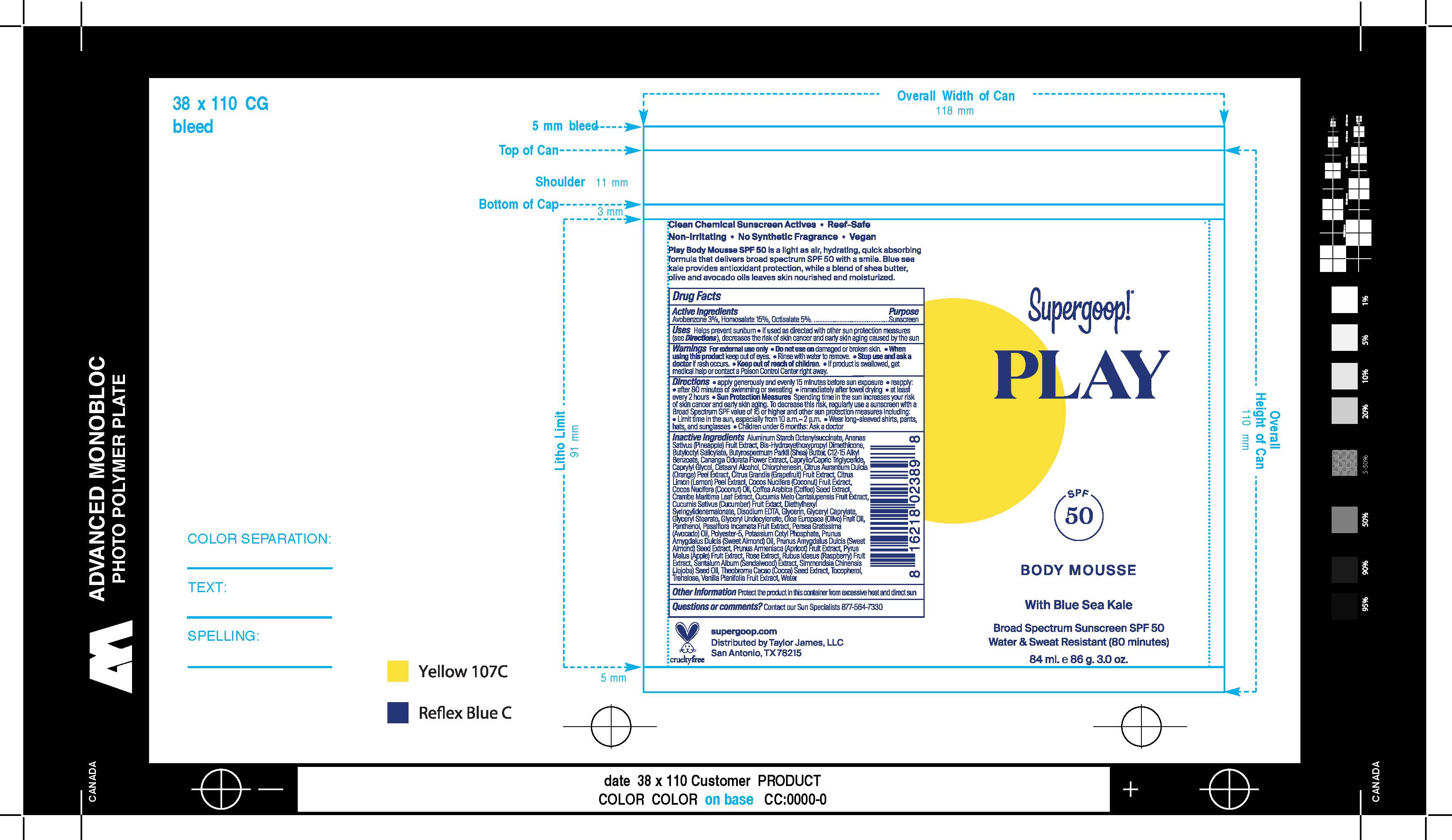
Label: PLAY BODY MOUSSE WITH BLUE SEA KALE BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate cream
- NDC Code(s): 75936-227-01, 75936-227-02
- Packager: Supergoop, LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 31, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Apply generously and evenly 15 minutes before sun exposure
Use a water-resistant sunscreen if swimming or sweating
Reapply at least every 2 hours.
Sun Protection Measures Spending time in the sun increases your risk of
skin cancer and early skin aging. To decrease this risk, regularly use a
sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sun
protection measures including: • limit your time in the sun, especially from 10
a.m. – 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses •
Children under 6 months of age: ask a doctor. -
INACTIVE INGREDIENT
Inactive Ingredients
Aluminum Starch Octenylsuccinate, Ananas Sativus (Pineapple) Fruit Extract, Bis-Hydroxyethoxypropyl Dimethicone,Butyloctyl Salicylate, Butyrospermum Parkii (Shea) Butter, C12-15 Alkyl Benzoate, Cananga Odorata Flower Extract, Caprylic/Capric Triglyceride, Caprylyl Glycol, Cetearyl Alcohol, Chlorphenesin, Citrus Aurantium Dulcis (Orange) Peel Extract, Citrus Grandis (Grapefruit) Fruit Extract, Citrus Limon (Lemon) Peel Extract, Cocos Nucifera (Coconut) Fruit Extract, Cocos Nucifera (Coconut) Oil, Coffea Arabica (Coffee) Seed Extract, Crambe Maritima Leaf Extract, Cucumis Melo Cantalupensis Fruit Extract, Cucumis Sativus (Cucumber) Fruit Extract, Diethylhexyl Syringylidenemalonate, Disodium EDTA, Glycerin, Glyceryl Caprylate, Glyceryl Stearate, Glyceryl Undecylenate, Olea Europaea (Olive) Fruit Oil, Panthenol, Passiflora Incarnata Fruit Extract, Persea Gratissima (Avocado) Oil, Polyester-5, Potassium Cetyl Phosphate, Prunus Amygdalus Dulcis (Sweet Almond) Seed Extract, Prunus Armeniaca (Apricot) Fruit Extract, Pyrus Malus (Apple) Fruit Extract, Rose Extract, Rubus Idaeus (Raspberry) Fruit Extract, Santalum Album (Sandalwood) Extract, Simmondsia Chinensis (Jojoba) Seed Oil, Theobroma Cacao (Cocoa) Seed Extract, Tocopherol, Trehalose, Vanilla Planifolia Fruit Extract, Water
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PLAY BODY MOUSSE WITH BLUE SEA KALE BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-227 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 15 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength AVOCADO OIL (UNII: 6VNO72PFC1) APPLE (UNII: B423VGH5S9) COCONUT (UNII: 3RT3536DHY) ARABICA COFFEE BEAN (UNII: 3SW678MX72) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) GLYCERYL 1-UNDECYLENATE (UNII: B68LJT9544) VANILLA BEAN (UNII: Q74T35078H) CANTALOUPE (UNII: 8QF5D5H6UH) CUCUMBER (UNII: YY7C30VXJT) OLIVE OIL (UNII: 6UYK2W1W1E) ALMOND (UNII: 3Z252A2K9G) RASPBERRY (UNII: 4N14V5R27W) TREHALOSE (UNII: B8WCK70T7I) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) POLYESTER-5 (TG-48) (UNII: 791E7S99Q2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) SANDALWOOD (UNII: 3641YW25N2) COCOA (UNII: D9108TZ9KG) PUMMELO (UNII: ET1TN5W71X) SHEA BUTTER (UNII: K49155WL9Y) LEMON PEEL (UNII: 72O054U628) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CHLORPHENESIN (UNII: I670DAL4SZ) ORANGE PEEL (UNII: TI9T76XD44) GLYCERIN (UNII: PDC6A3C0OX) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) CANANGA ODORATA FLOWER (UNII: 76GTF6Z97M) PANTHENOL (UNII: WV9CM0O67Z) PASSIFLORA INCARNATA FRUIT (UNII: SF206I8G4P) APRICOT (UNII: 269CJD5GZ9) JOJOBA OIL (UNII: 724GKU717M) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COCONUT OIL (UNII: Q9L0O73W7L) CRAMBE MARITIMA LEAF (UNII: NO0DI2V62B) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALMOND OIL (UNII: 66YXD4DKO9) PINEAPPLE (UNII: 2A88ZO081O) BIS-HYDROXYETHOXYPROPYL DIMETHICONE (37 CST) (UNII: 7K226YI89Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-227-01 84 mL in 1 CAN; Type 0: Not a Combination Product 04/02/2020 2 NDC:75936-227-02 181 mL in 1 CAN; Type 0: Not a Combination Product 04/02/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/02/2020 Labeler - Supergoop, LLC (117061743)