Label: ALCOHOL PREP PADS- isopropyl alcohol swab
- NDC Code(s): 63517-600-01, 63517-600-02, 63517-600-50
- Packager: Cardinal Health 200, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 17, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions for use
- Inactive Ingredient
-
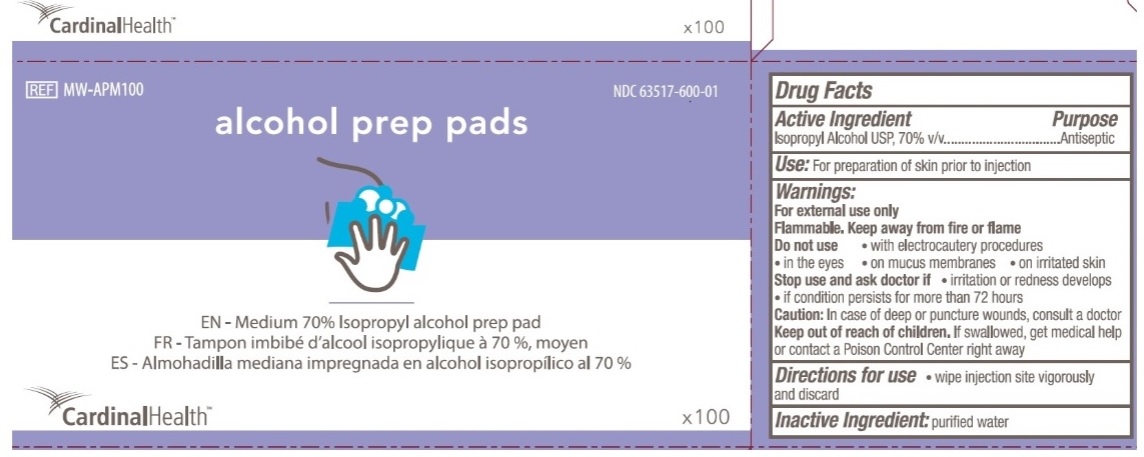
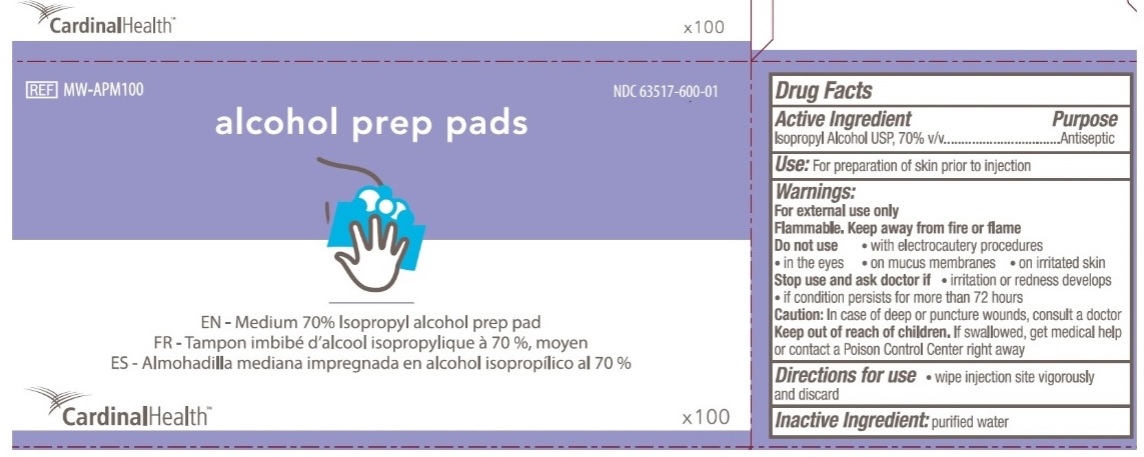
Principal Display Panel
CardinalHealth
NDC 63517-600-01
REF MW-APM100
alcohol prep pads
EN - Medium 70% Isopropyl alcohol prep pad
FR - Tampon imbibe d'alcool isopropylique a 70%, moyen
ES - Almohadilla mediana impregnada en alcohol isopropilico al 70%
CardinalHealth
X100
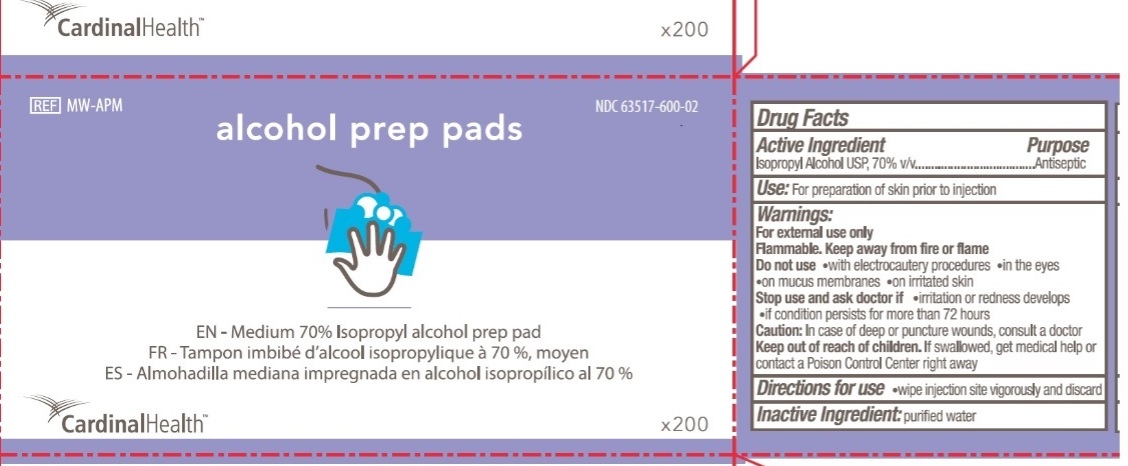
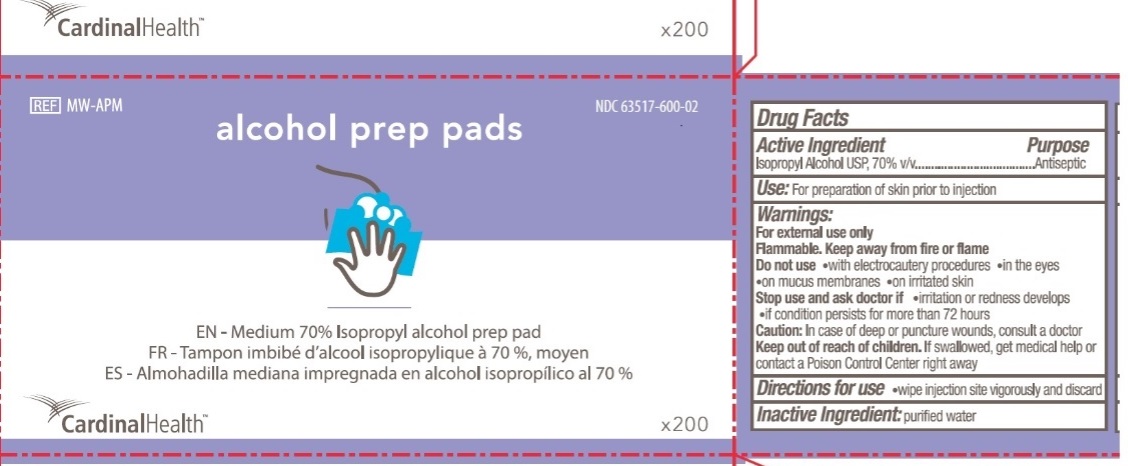
CardinalHealth
NDC 63517-600-02
REF MW-APM
alcohol prep pads
EN - Medium 70% Isopropyl alcohol prep pad
FR - Tampon imbibe d'alcool isopropylique a 70%, moyen
ES - Almohadilla mediana impregnada en alcohol isopropilico al 70%
CardinalHealth
X200
CardinalHealth
NDC 63517-600-50
REF MW-APM50
alcohol prep pads
EN - Medium 70% Isopropyl alcohol prep pad
FR - Tampon imbibe d'alcool isopropylique a 70%, moyen
ES - Almohadilla mediana impregnada en alcohol isopropilico al 70%
CardinalHealth
X50

-
INGREDIENTS AND APPEARANCE
ALCOHOL PREP PADS
isopropyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63517-600 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63517-600-50 50 in 1 CARTON 02/08/2016 1 1 mL in 1 PACKET; Type 0: Not a Combination Product 2 NDC:63517-600-01 100 in 1 CARTON 02/08/2016 2 1 mL in 1 PACKET; Type 0: Not a Combination Product 3 NDC:63517-600-02 200 in 1 CARTON 02/08/2016 3 1 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/08/2016 Labeler - Cardinal Health 200, Inc. (961027315) Registrant - Cardinal Health 200, Inc. (961027315)