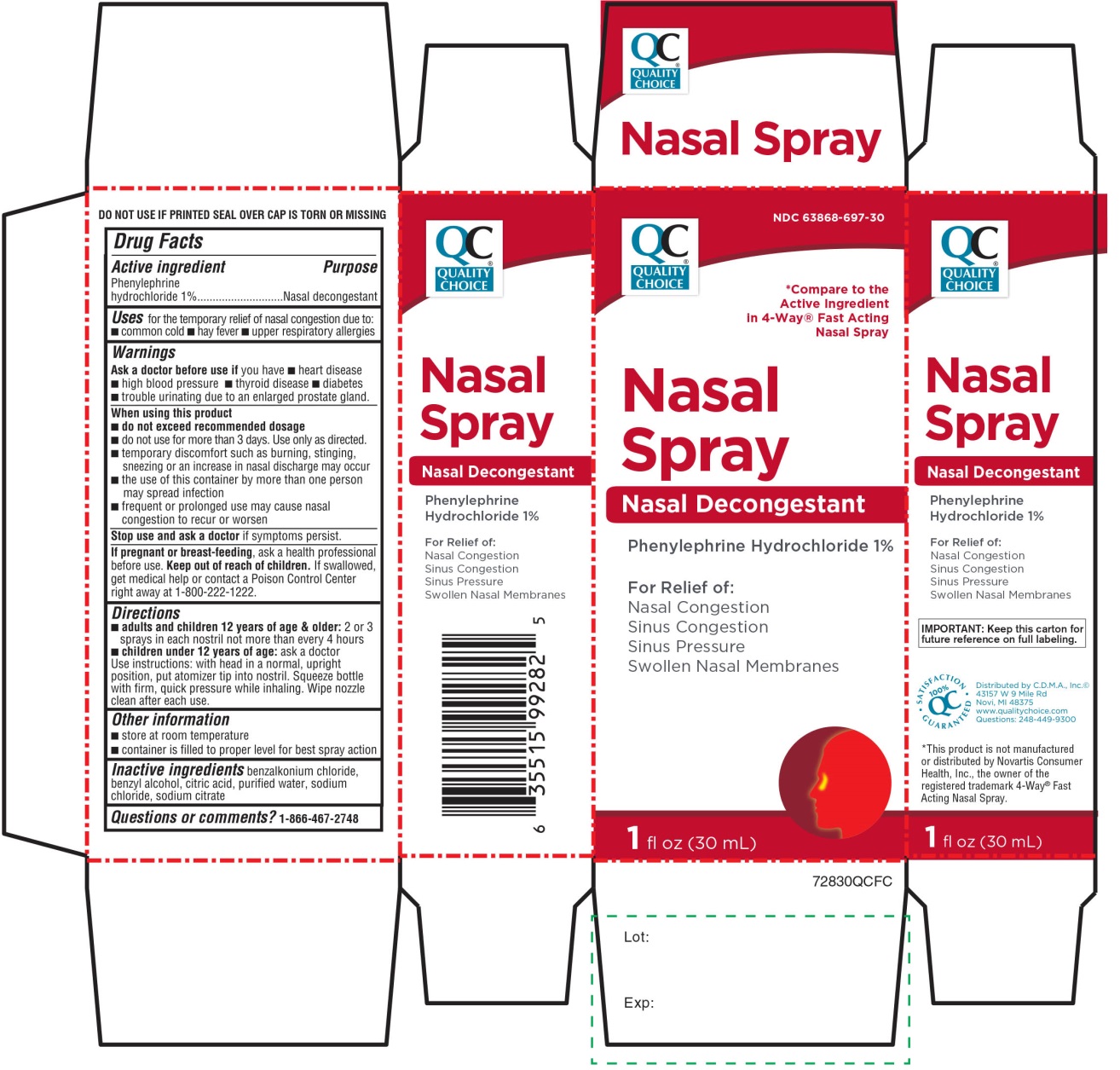
Label: QUALITY CHOICE- phenylephrine hydrochloride solution/ drops
- NDC Code(s): 63868-697-30
- Packager: CHAIN DRUG MARKETING ASSOCIATION
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 9, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
When using this product
- •
- do not use more than directed
- •
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- •
- temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge may occur
- •
- use of this container by more than one person may spread infection
-
Directions
- •
- adults and children 12 years of age and older: 2 or 3 drops in each nostril not more often than every 4 hours
- •
- children under 12 years of age: ask a doctor
Use instructions: with head in a normal, upright position, put atomizer tip into nostril. Squeeze bottle with firm, quick pressure while inhaling. Wipe nozzle clean after each use.
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
NDC# 63868-697-30
*Compare to Active Ingredient in 4-Way® Fast Acting Nasal Spray
Nasal Spray
Nasal Decongestant
PHENYLEPHRINE HCl 1%
For Relief of:
Nasal Congestion
Sinus Congestion
Sinus Pressure
Swollen Nasal Membranes
100% QC SATISFACTION GUARANTEED
1 FL OZ (30 mL)
Distributed by: C.D.M.A. Inc. ©
43157 W 9 Mile Rd
Novi, MI 48375
Questions: 248-449-9300
*This product is not manufactured or distributed by Novartis Consumer Health, Inc. the owner of the registered trademark 4-Way® Fast Acting Nasal Spray.
-
INGREDIENTS AND APPEARANCE
QUALITY CHOICE
phenylephrine hydrochloride solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-697 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-697-30 1 in 1 CARTON 12/18/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/18/2018 Labeler - CHAIN DRUG MARKETING ASSOCIATION (011920774)