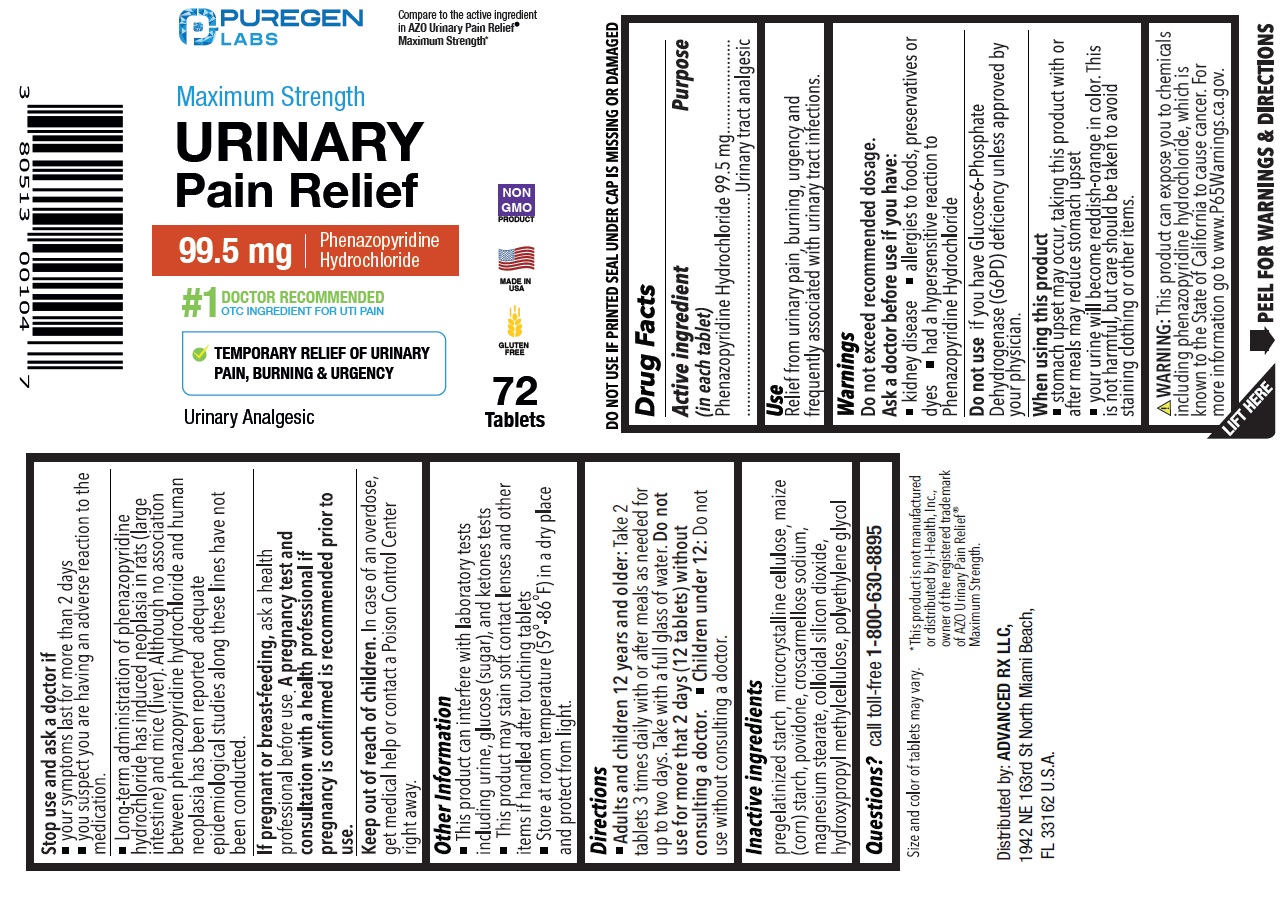
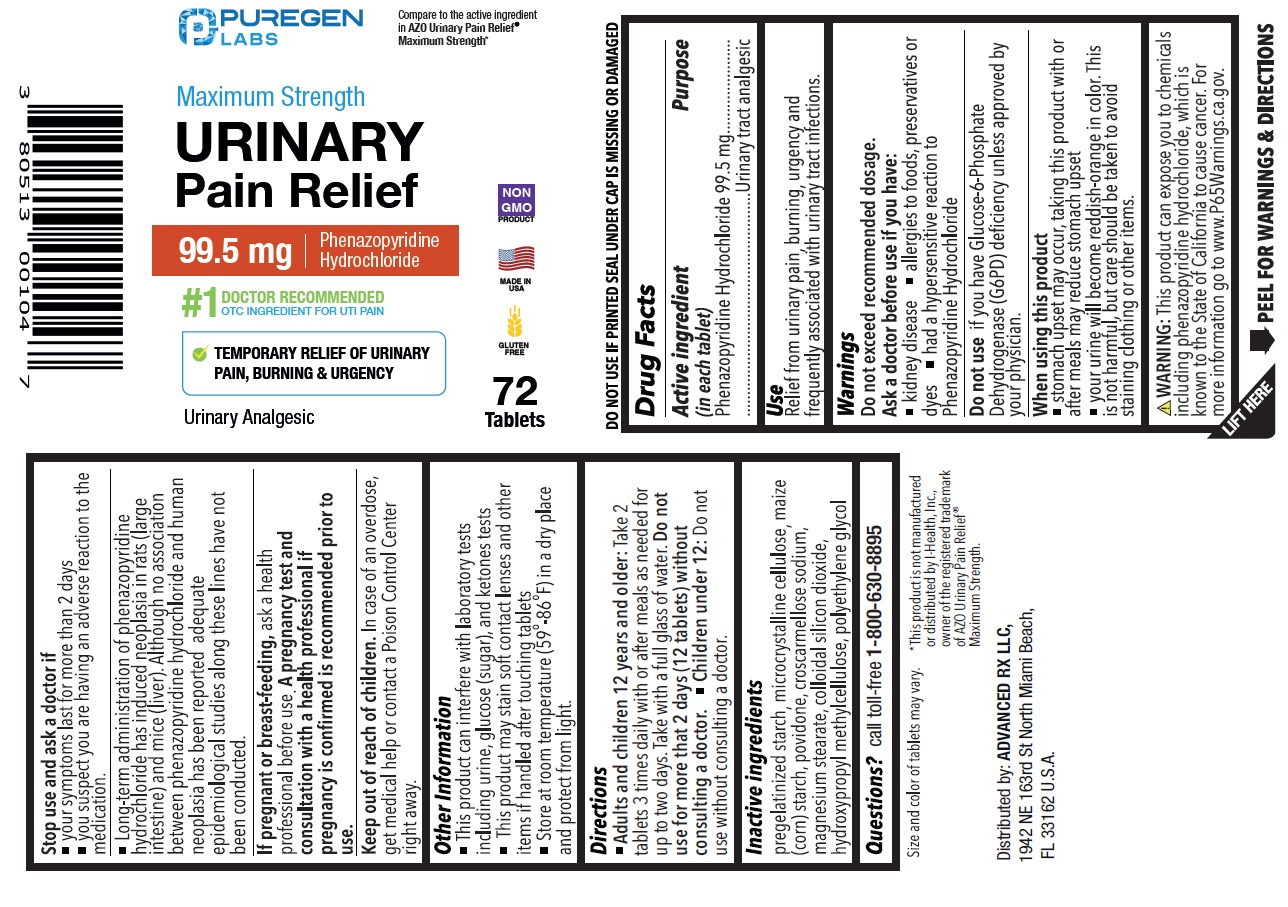
Label: MAXIMUM STRENGTH URINARY PAIN RELIEF- phenazopyridine hydrochloride tablet
- NDC Code(s): 80513-517-72
- Packager: Advanced Rx LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each tablet)
- Purpose
- Use
-
Warnings
Do not exceed recommended dosage.
Ask a doctor before use if you have:
• kidney disease
• allergies to foods, preservatives or dyes
• had a hypersensitive reaction to Phenazopyridine Hydrochloride
Do not useif you have Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency unless approved by your physician.
When using this product
• stomach upset may occur, taking this product with or after meals may reduce stomach upset
• your urine will become reddish-orange in color. This is not harmful, but care should be taken to avoid staining clothing or other items.
Stop use and ask a doctor if
• your symptoms last for more than 2 days
• you suspect you are having an adverse reaction to the medication.
• Long-term administration of phenazopyridine hydrochloride has induced neoplasia in rats (large intestine) and mice (liver). Although no association between phenazopyridine hydrochloride and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
If pregnant or breastfeeding, ask a health professional before use. A pregnancy test and consultation with a health professional if pregnancy is confirmed is recommended prior to use.
- Other information
- Directions
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
Compare to the active ingredient in AZO Urinary Pain Relief ®Maximum Strength*
NDC 80513-517-72
Maximum Strength Urinary Pain Relief
99.5 mg Phenazopyridine Hydrochloride
#1 DOCTOR RECOMMENDED OTC INGREDIENT FOR UTI PAIN
TEMPORARY RELIEF OF URINARY PAIN, BURNING & URGENCY
Urinary Analgesic
72 Tablets
DO NOT USE IF PRINTED SEAL UNDER CAP IS MISSING OR DAMAGED
Size and color of tablet may very.
WARNING: This product can expose you to chemicals including phenazopyridine hydrochloride, which is known to the State of California to cause cancer. For more information go to www.P65Warnings.ca.gov.
*This product is not manufactured or distributed by I-Health, Inc., owner of the registered trademark of AZO Urinary Pain Relief ®Maximum Strength
Distributed by:
ADVANCED RX LLC,
1942 NE 163rd St North Miami Beach,
FL 33162 U.S.A.
MADE IN USA
-
INGREDIENTS AND APPEARANCE
MAXIMUM STRENGTH URINARY PAIN RELIEF
phenazopyridine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80513-517 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENAZOPYRIDINE HYDROCHLORIDE (UNII: 0EWG668W17) (PHENAZOPYRIDINE - UNII:K2J09EMJ52) PHENAZOPYRIDINE HYDROCHLORIDE 99.5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color brown Score no score Shape ROUND Size 7mm Flavor Imprint Code SP9 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80513-517-72 72 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/2024 Labeler - Advanced Rx LLC (042795108) Establishment Name Address ID/FEI Business Operations INVAHEALTH INC. 116840615 manufacture(80513-517)