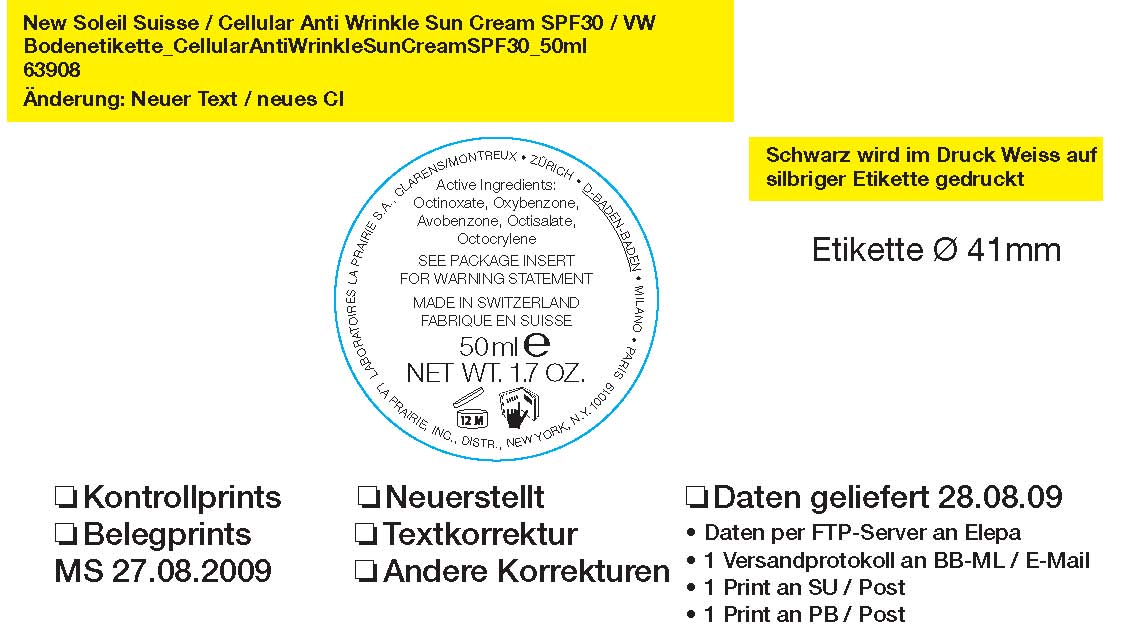
Label: LA PRAIRIE SWITZERLAND ANTI-WRINKLE SUNSCREEN SPF 30- avobenzone, octinoxate, octisalate, octocrylene, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 59614-411-01, 59614-411-02 - Packager: Juvena Produits de Beaute GMBH
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 1, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients:
Octinoxate 7.5%
Oxybenzone 5%
Avobenzone 3%
Octisalate 2.6%
Octocrylene 2.4%
Warnings: For external use only. Avoid contact with eyes. Discontinue use if irritation occurs. Keep out of reach of children. Do not use on children. Contains: Oxybenzone (Benzophenone-3)
Ingredients: Water (Aqua), Ethylhexyl Methoxycinnimate, Benzophenone-3, Cyclomethicone, Hexyldecanol, Butylene Methoxydibenzoylmethane, Ethylhexyl Salicylate, PPG-3 Benzyl Ether Myristate, Coco-Caprylate/Caprate, Octocrylene, C12-15 Alkyl Benzoate, Isohexadecane, Butylene Glycol, Octyldodecanol, PEG-100 Stearate, Glyceryl Stearate, Styrene/Actylates Copolymer, Phenyl Trimethicone, Cetearyl Alcohol, Butyrospermum Parkii (Shea Butter), Glycoproteins, Panax Ginseng Root Extract, Equisetum Arvense (Horsetail) Extract, Tocopheryl Acetate, Retinyl Palmitate, Aloe Barbadensis Leaf Juice, RNA, Pyrus Communis (Pear) Fruit Extract, Camellia Sinensis Leaf Extract, Caprylic/Capric Triglyceride, Macadamia Ternifolia Seed Oil, Ethylhexylglycerin, Caprylyl Glycol, Beeswax (Cera Alba), Arginine, Pyridoxine HCL, Lecithin, Hibisus Sabdariffa Flower Extract, Ferula Assa Foetida Root Extract, Tyrosine, Ceteareth-20, Sodium Carbomer, Glycerin, Ursolic Acid, Sodium Hyaluronate, Glycine Soja (soybean) Oil, laminaria Ochroleuca Extract, Lavandula Angustifolia (lavender) extract, sodium polyacrylate, mannitol, disodium adenosine triphosphate, PEG-8 Laurate, 1,2-Hexanediol, histidine HCL, Dimethicone Crosspolymer-3, disodium EDTA, phenylalanine, Glyceryl Polyacrylate, Chamomilla Recutita (Matricaria) Flower Extract, Phospholipids, Oleic Acid, Linoleic Acid, Linolenic Acid, Xanthan Gum, Dipotassium Glycyrrihizate, Phytosphingosine, Cholesterol, Ceramide 3, Atelocollagen, Alcohol Denat., Sodium Chondroitin Sulfate, Ubiquinone, Thiotic Acid, Palmitic Acid, Micrococcus Lysate, Beta-Carotene, Fragrance (Parfum), Linalool, Hexyl Cinnamal, Hydroxycitronellal, Alpha-Isomethl Ionone, Geraniol, Citronellol, Benzyl Benzoate, Butylphenyl Methylpropional, Evernia Furfuracea (Treemoss) Extract, Chlorphenesin, Phenoxyethanol, Methylparaben, Yellow 5 (CI 19140), Orange 4 (CI 15510)
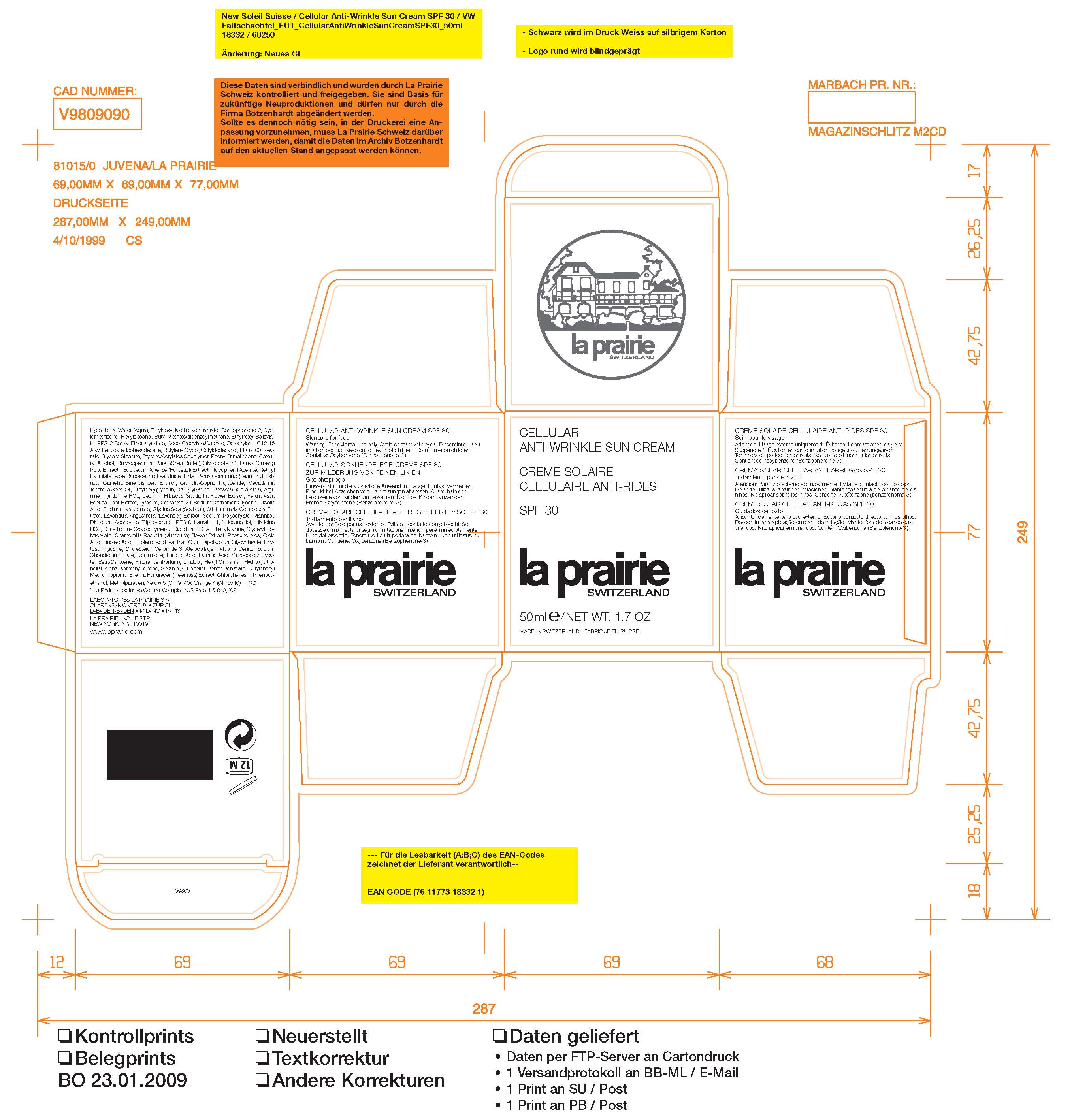
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LA PRAIRIE SWITZERLAND ANTI-WRINKLE SUNSCREEN SPF 30
avobenzone, octinoxate, octisalate, octocrylene, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59614-411 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 mL in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mL in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 2.6 mL in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.4 mL in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 5 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59614-411-02 1 in 1 BOX 1 NDC:59614-411-01 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/01/2009 Labeler - Juvena Produits de Beaute GMBH (315053785) Registrant - Juvena Produits de Beaute GMBH (315053785) Establishment Name Address ID/FEI Business Operations Temmemtec Ag 480586411 manufacture Establishment Name Address ID/FEI Business Operations Trichema Ag 480006600 manufacture