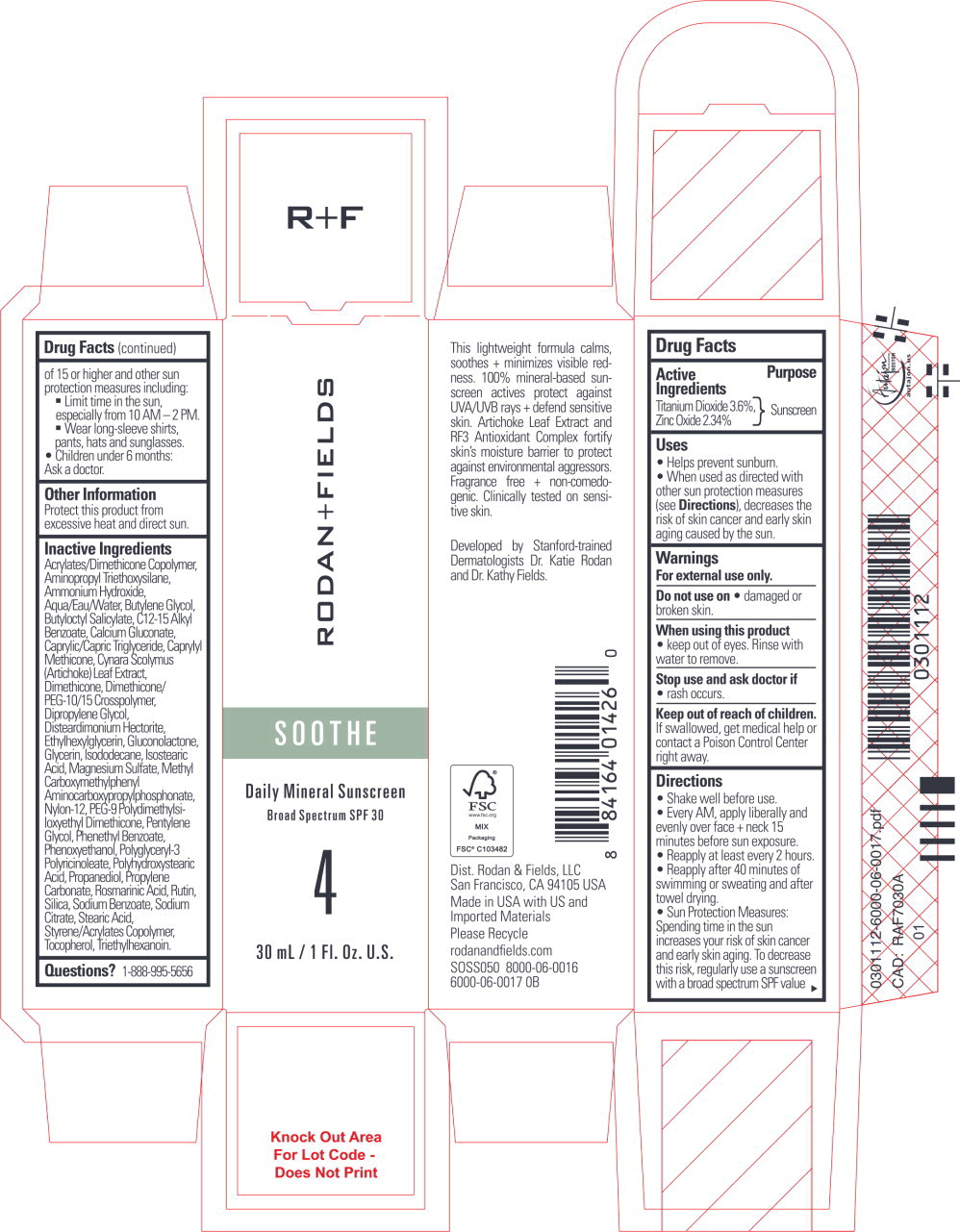
Label: SOOTHE DAILY MINERAL SUNSCREEN- titanium dioxide, zinc oxide lotion
- NDC Code(s): 14222-2440-1, 14222-2440-2, 14222-2440-3
- Packager: Rodan & Fields
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 30, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
-
Uses
- Helps prevent sunburn.
- When used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Shake well before use.
- Every AM, apply liberally and evenly over face + neck 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Reapply after 40 minutes of swimming or sweating and after towel drying.
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 AM - 2 PM.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Children under 6 months:
Ask a doctor.
- Other Information
-
Inactive Ingredients
Acrylates/Dimethicone Copolymer, Aminopropyl Triethoxysilane, Ammonium Hydroxide, Aqua/Eau/Water, Butylene Glycol, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Calcium Gluconate, Caprylic/Capric Triglyceride, Caprylyl Methicone, Cynara Scolymus (Artichoke) Leaf Extract, Dimethicone, Dimethicone/PEG-10/15 Crosspolymer, Dipropylene Glycol, Disteardimonium Hectorite, Ethylhexylglycerin, Gluconolactone, Glycerin, Isododecane, Isostearic Acid, Magnesium Sulfate, Methyl Carboxymethylphenyl Aminocarboxypropylphosphonate, Nylon-12, PEG-9 Polydimethylsiloxyethyl Dimethicone, Pentylene Glycol, Phenethyl Benzoate, Phenoxyethanol, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Propanediol, Propylene Carbonate, Rosmarinic Acid, Rutin, Silica, Sodium Benzoate, Sodium Citrate, Stearic Acid, Styrene/Acrylates Copolymer, Tocopherol, Triethylhexanoin.
- Questions?
- Principal Display Panel - 30 mL Carton Label
- Principal Display Panel - 30 mL Tube Label
-
INGREDIENTS AND APPEARANCE
SOOTHE DAILY MINERAL SUNSCREEN
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2440 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.036 g in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0234 g in 1 mL Inactive Ingredients Ingredient Name Strength 3-(TRIETHOXYSILYL)PROPYLAMINE (UNII: L8S6UBW552) AMMONIA (UNII: 5138Q19F1X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CALCIUM GLUCONATE (UNII: SQE6VB453K) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CYNARA SCOLYMUS LEAF (UNII: B71UA545DE) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) DIPROPYLENE GLYCOL (UNII: E107L85C40) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLYCERIN (UNII: PDC6A3C0OX) ISODODECANE (UNII: A8289P68Y2) ISOSTEARIC ACID (UNII: X33R8U0062) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) NYLON-12 (UNII: 446U8J075B) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) PENTYLENE GLYCOL (UNII: 50C1307PZG) PHENETHYL BENZOATE (UNII: 0C143929GK) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PROPANEDIOL (UNII: 5965N8W85T) PROPYLENE CARBONATE (UNII: 8D08K3S51E) ROSMARINIC ACID (UNII: MQE6XG29YI) RUTIN (UNII: 5G06TVY3R7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) STEARIC ACID (UNII: 4ELV7Z65AP) TOCOPHEROL (UNII: R0ZB2556P8) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2440-1 1 in 1 CARTON 03/09/2021 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:14222-2440-2 1 in 1 CARTON 06/01/2021 2 7 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:14222-2440-3 2 mL in 1 PACKET; Type 0: Not a Combination Product 04/12/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/09/2021 Labeler - Rodan & Fields (051659584)