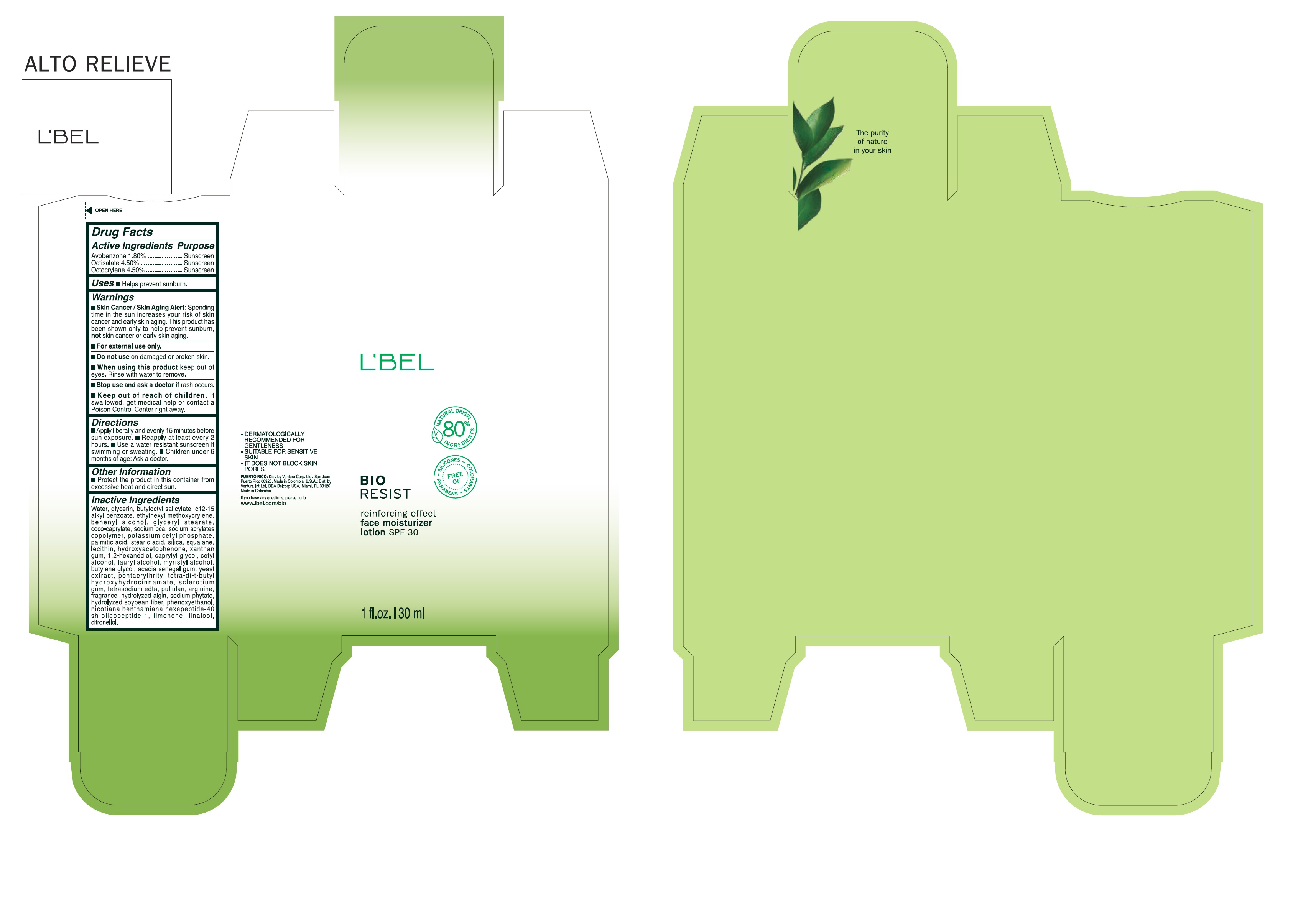
Label: BIORESIST REINFORCING EFFECT FACE MOISTURIZER SPF 30- avobenzone, octisalate, octocrylene cream
- NDC Code(s): 14141-142-01, 14141-142-02
- Packager: BEL STAR S A
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 29, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin .
When using this product keep out of eyes. Rinse with water to remove.
- DOSAGE & ADMINISTRATION
- Other Information
-
INACTIVE INGREDIENT
Inactive ingredients
Water, glycerin, butyloctyl salicylate, c12-15 alkyl benzoate, ethylhexyl methoxycrylene, behenyl alcohol, glyceryl stearate, coco-caprylate, sodium pca, sodium acrylates copolymer, potassium cetyl phosphate, palmitic acid, stearic acid, silica, squalane, lecithin, hydroxyacetophenone, xanthan gum, 1,2- hexandiol, caprylyl glycol, cetyl alcohol, lauryl alcohol, myristyl alcohol, butylene glycol, acacia senegal gum, yeast extract, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, sclerotium gum, tetrasodium edta, pullulan, arginine, fragrance, hydrolyzed algin, sodium phytate, hydrolyzed soybean fiber, phenoxyethanol, nicotiana benthamiana hexapeptide-40 sh-oligopeptide-1, limonene, linalool, citronellol.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIORESIST REINFORCING EFFECT FACE MOISTURIZER SPF 30
avobenzone, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-142 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4.5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.8 g in 100 mL Inactive Ingredients Ingredient Name Strength MYRISTYL ALCOHOL (UNII: V42034O9PU) ACACIA (UNII: 5C5403N26O) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DOCOSANOL (UNII: 9G1OE216XY) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) COCO-CAPRYLATE (UNII: 4828G836N6) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) ARGININE (UNII: 94ZLA3W45F) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) PALMITIC ACID (UNII: 2V16EO95H1) BETASIZOFIRAN (UNII: 2X51AD1X3T) EDETATE SODIUM (UNII: MP1J8420LU) GLYCERIN (UNII: PDC6A3C0OX) PULLULAN (UNII: 8ZQ0AYU1TT) PHYTATE SODIUM (UNII: 88496G1ERL) PHENOXYETHANOL (UNII: HIE492ZZ3T) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) LAURYL ALCOHOL (UNII: 178A96NLP2) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SQUALANE (UNII: GW89575KF9) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) XANTHAN GUM (UNII: TTV12P4NEE) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CETYL ALCOHOL (UNII: 936JST6JCN) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-142-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2019 2 NDC:14141-142-02 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/21/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 12/21/2019 Labeler - BEL STAR S A (880160197) Establishment Name Address ID/FEI Business Operations BEL STAR S A 880160197 manufacture(14141-142)