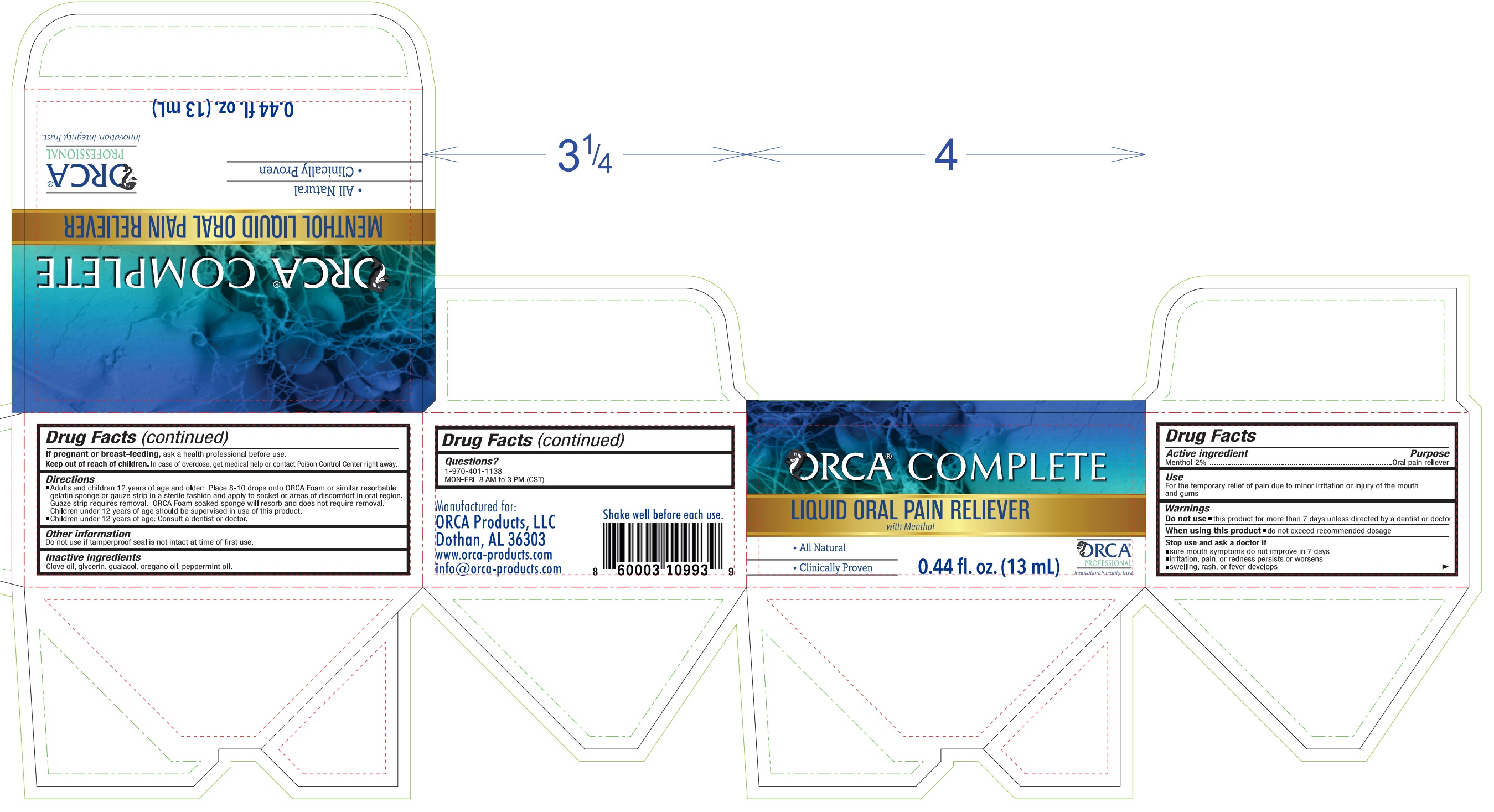
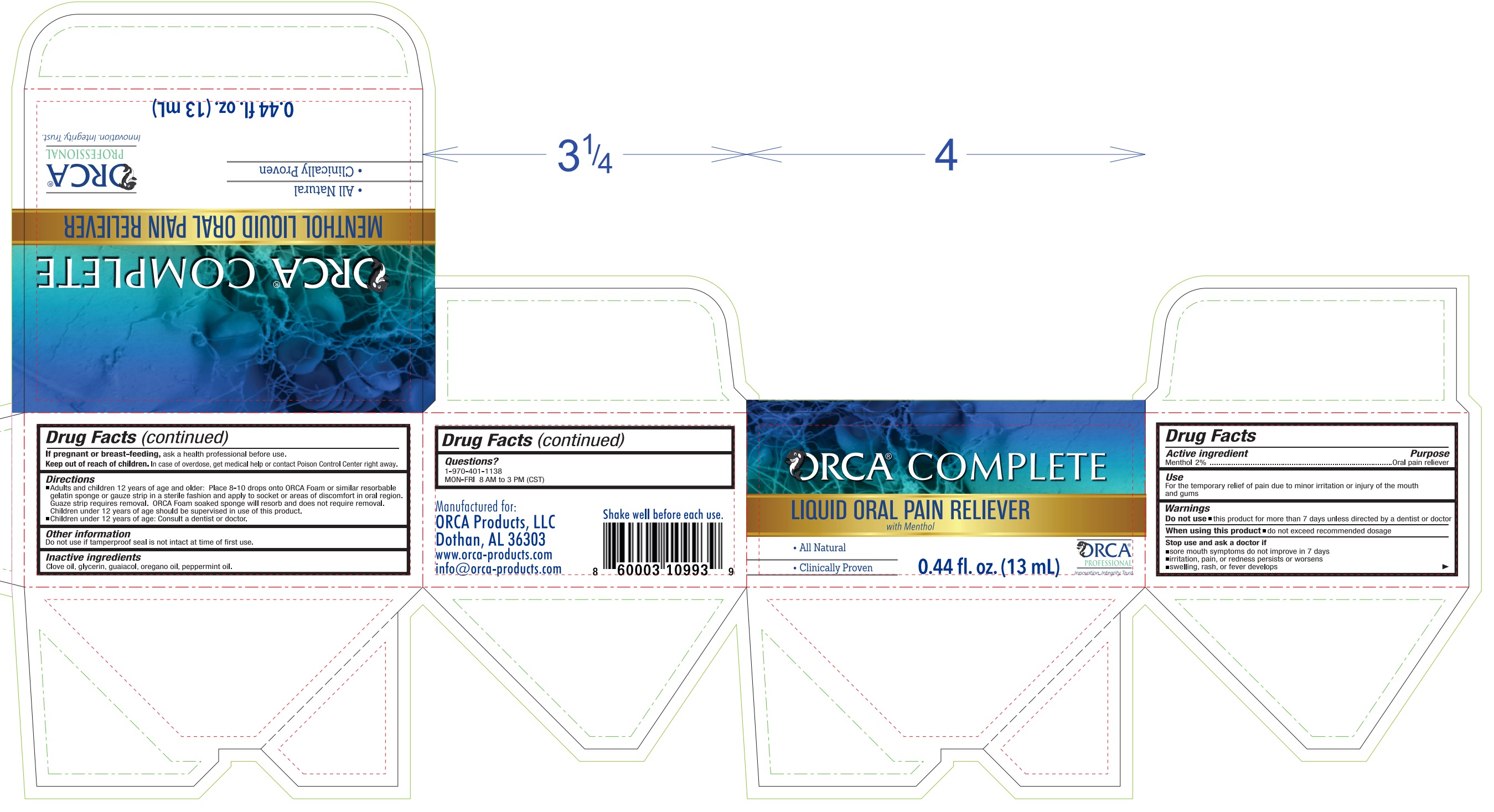
Label: ORCA COMPLETE MENTHOL PAIN RELIEVER- menthol liquid
- NDC Code(s): 82939-000-01, 82939-000-02
- Packager: ORCA Products, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 8, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- Adults and children 12 years of age and older: Place 8-10 drops onto ORCA Foam or similar resorbable gelatin sponge or gauze strip in a sterile fashion and apply to areas of discomfort in oral region. Guaze strip requires removal. Orca Foam soaked sponge will resorb and does not require removal.
- Children under 12 years of age should be supervised in use of this product.
- Children under 12 years of age: Consult a dentist or doctor.
- Other information
- Inactive ingredients
- Questions?
- Package Labeling: 82939-000-02
-
INGREDIENTS AND APPEARANCE
ORCA COMPLETE MENTHOL PAIN RELIEVER
menthol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82939-000 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength CLOVE OIL (UNII: 578389D6D0) GLYCERIN (UNII: PDC6A3C0OX) GUAIACOL (UNII: 6JKA7MAH9C) OREGANO LEAF OIL (UNII: 7D0CGR40U1) PEPPERMINT OIL (UNII: AV092KU4JH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82939-000-01 1 in 1 BOX 09/15/2022 08/31/2025 1 10 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC:82939-000-02 1 in 1 BOX 09/15/2023 2 13 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 09/15/2022 Labeler - ORCA Products, LLC (117716283) Registrant - ORCA Products, LLC (117716283)