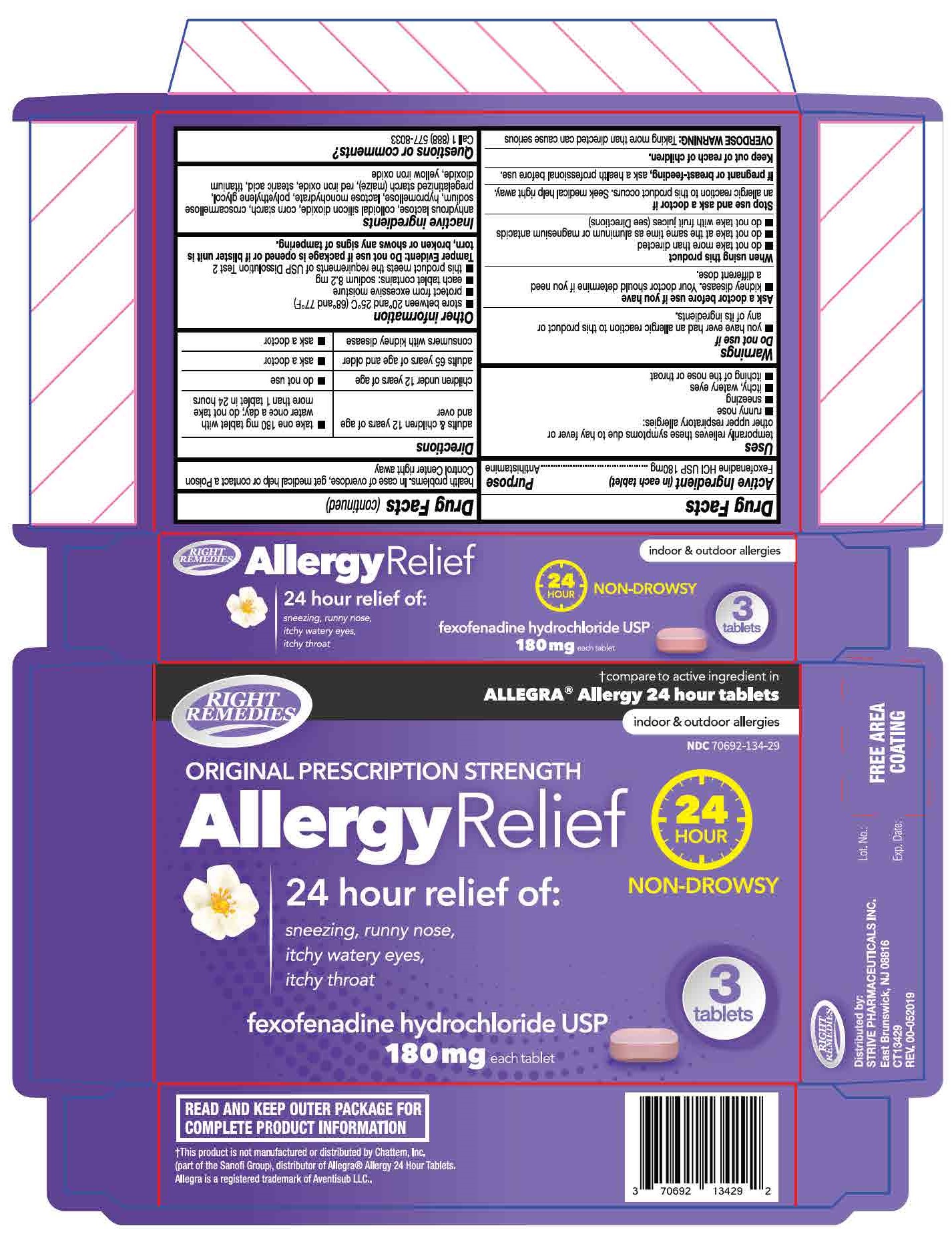
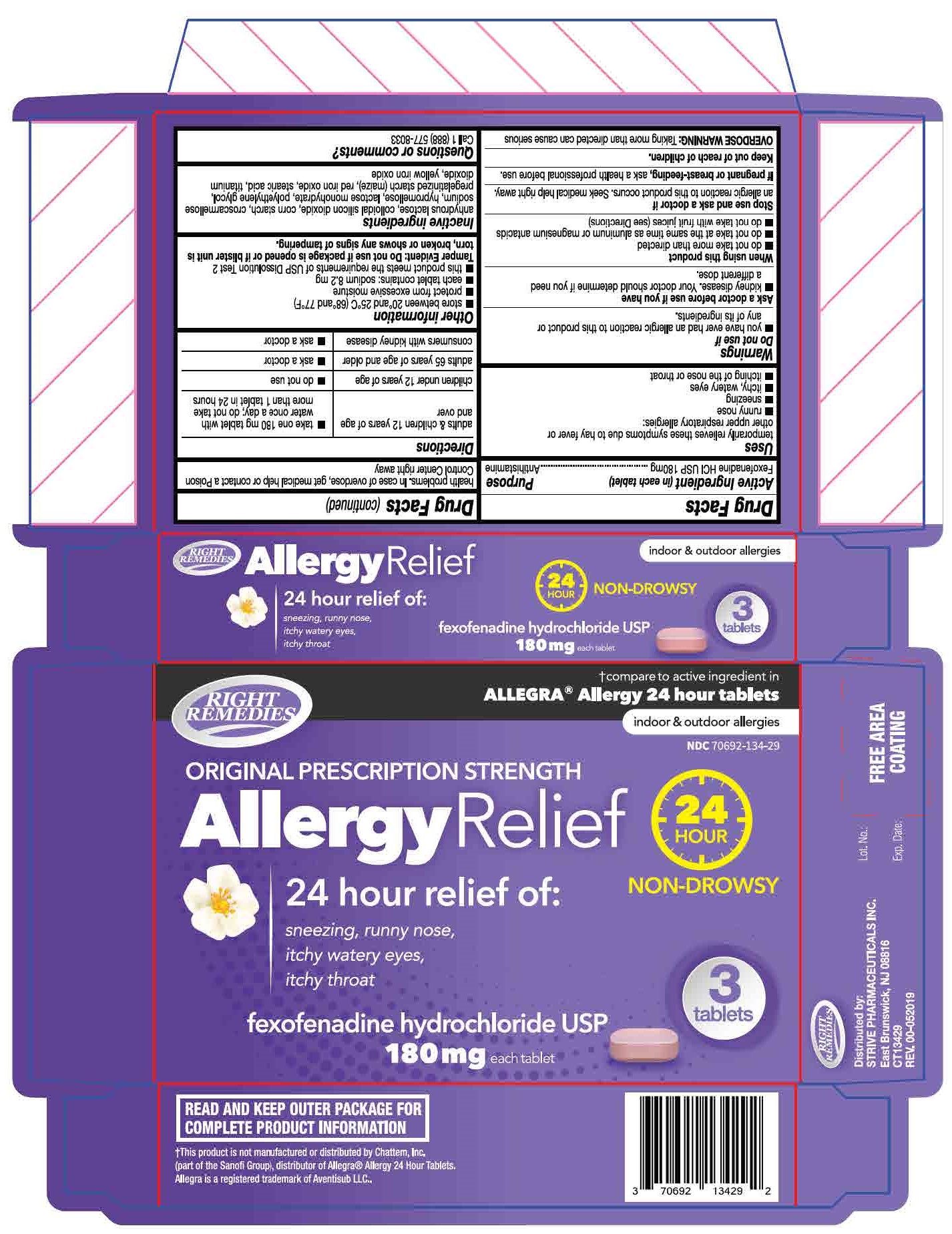
Label: FEXOFENADINE HYDROCHLORIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 70692-134-29 - Packager: Strive Pharmaceuticals Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 16, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
WARNINGS
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
- kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding,
ask a health professional before use
Keep out of reach of children
Overdose warning: Taking more than directed can cause serious health problems. In case of overdose, get medical help or contact a Poison Control Center right away.
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
-
OTHER SAFETY INFORMATION
- store at room temperature between 20 ºC - 25 ºC (68 ºF - 77 ºF)
- protect from excessive moisture
- each tablet contains: sodium 8.2 mg
- this product meets the requirements of USP Dissolution Test 2
Tamper Evident: Do not use if package is opened or if blister unite is torn broken or shows any signs of tampering.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70692-134 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) FERRIC OXIDE RED (UNII: 1K09F3G675) STEARIC ACID (UNII: 4ELV7Z65AP) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Product Characteristics Color pink Score no score Shape CAPSULE Size 18mm Flavor Imprint Code SG;202 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70692-134-29 3 in 1 BLISTER PACK; Type 0: Not a Combination Product 07/22/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211075 07/22/2019 Labeler - Strive Pharmaceuticals Inc (080028013)