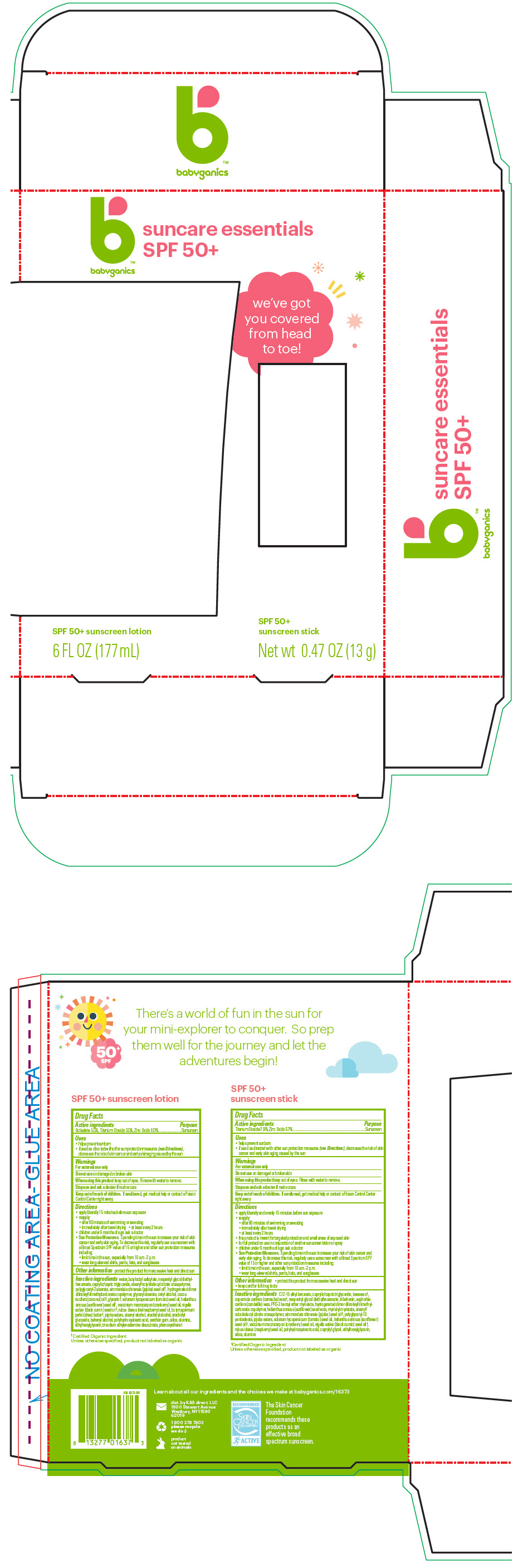
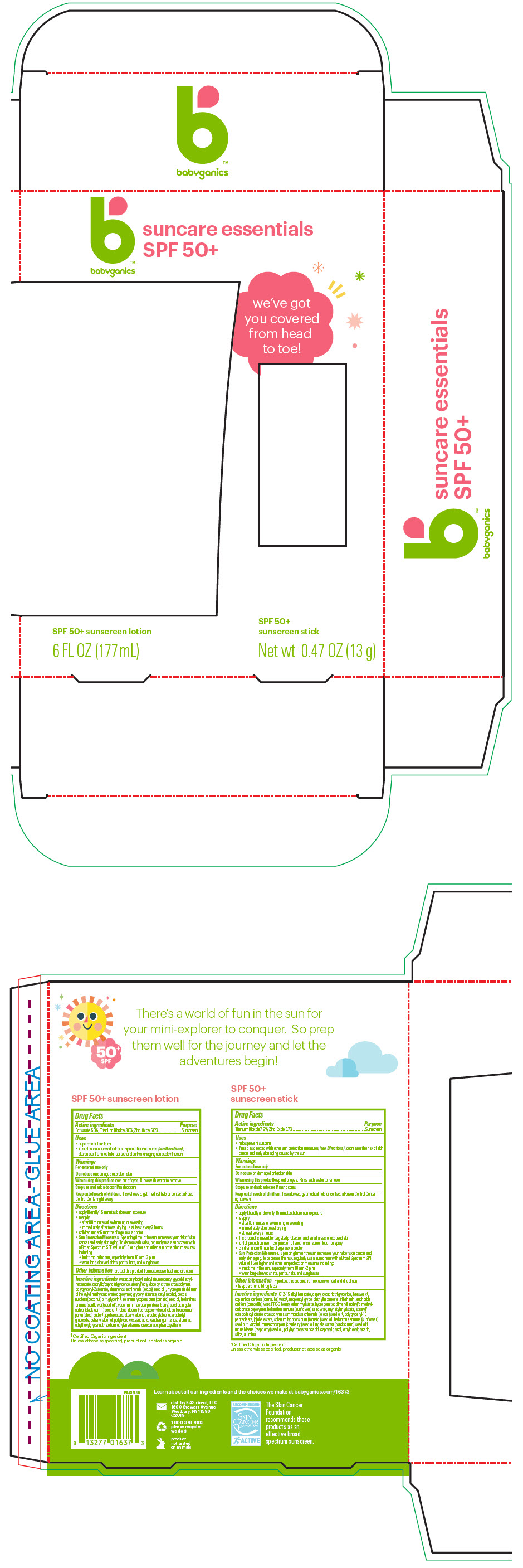
Label: SUNCARE ESSENTIALS- titanium dioxide, octisalate, and zinc oxide kit
- NDC Code(s): 59062-1000-6, 59062-1200-1, 59062-5100-1
- Packager: KAS Direct LLC dba BabyGanics
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 4, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other information
-
Inactive ingredients
water, butyloctyl salicylate, neopentyl glycol diethylhexanoate, caprylic/capric triglyceride, stearyl/octyldodecyl citrate crosspolymer, polyglyceryl-2 stearate, simmondsia chinensis (jojoba) seed oil1, hydrogenated dimer dilinoleyl/dimethylcarbonate copolymer, glyceryl stearate, cetyl alcohol, cocos nucifera (coconut) oil1, glycerin1, solanum lycopersicum (tomato) seed oil, helianthus annuus (sunflower) seed oil1, vaccinium macrocarpon (cranberry) seed oil, nigella sativa (black cumin) seed oil1, rubus idaeus (red raspberry) seed oil, butyrospermum parkii (shea) butter1, jojoba esters, stearyl alcohol, arachidyl alcohol, arachidyl glucoside, behenyl alcohol, polyhydroxystearic acid, xanthan gum, silica, alumina, ethylhexylglycerin, trisodium ethylenediamine disuccinate, phenoxyethanol
- 1
- Certified Organic Ingredient
Unless otherwise specified, product not labeled as organic
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally and evenly 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- this product is meant for targeted protection and small areas of exposed skin
- for full protection use in conjunction of another sunscreen lotion or spray
- children under 6 months of age: ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other information
-
Inactive ingredients
C12-15 alkyl benzoate, caprylic/capric triglyceride, beeswax2, copernicia cerifera (carnauba) wax2, neopentyl glycol diethylhexanoate, tribehenin, euphorbia cerifera (candelilla) wax, PPG-3 benzyl ether myristate, hydrogenated dimer dilinoleyl/dimethylcarbonate copolymer, helianthus annuus (sunflower) seed wax, myristyl myristate, stearyl/octadodecyl citrate crosspolymer, simmondsia chinensis (jojoba) seed oil2, polyglyceryl-10 pentaoleate, jojoba esters, solanum lycopersicum (tomato) seed oil, helianthus annuus (sunflower) seed oil2, vaccinium macrocarpon (cranberry) seed oil, nigella sativa (black cumin) seed oil2, rubus idaeus (raspberry) seed oil, polyhydroxystearic acid, caprylyl glycol, ethylhexylglycerin, silica, alumina
- 2
- Certified Organic Ingredient
Unless otherwise specified, product not labeled as organic
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Package
-
INGREDIENTS AND APPEARANCE
SUNCARE ESSENTIALS
titanium dioxide, octisalate, and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59062-5100 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59062-5100-1 1 in 1 PACKAGE 01/01/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 177 mL Part 2 1 APPLICATOR 13 g Part 1 of 2 SPF 50 PLUS SUNSCREEN
titanium dioxide, octisalate, and zinc oxide lotionProduct Information Item Code (Source) NDC:59062-1000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Butyloctyl Salicylate (UNII: 2EH13UN8D3) Neopentyl Glycol Diethylhexanoate (UNII: U68ZV6W62C) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Stearyl/Octyldodecyl Citrate Crosspolymer (UNII: PN88NW0KPK) Polyglyceryl-2 Stearate (UNII: 253MC0P0YV) CETYL ALCOHOL (UNII: 936JST6JCN) JOJOBA OIL (UNII: 724GKU717M) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERIN (UNII: PDC6A3C0OX) COCONUT OIL (UNII: Q9L0O73W7L) TOMATO SEED OIL (UNII: 7N87T9C06T) SUNFLOWER OIL (UNII: 3W1JG795YI) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CRANBERRY SEED OIL (UNII: 73KDS3BW5E) Nigella Sativa Seed Oil (UNII: CS4U38E731) RASPBERRY SEED OIL (UNII: 9S8867952A) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) SHEA BUTTER (UNII: K49155WL9Y) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) Stearyl Alcohol (UNII: 2KR89I4H1Y) Arachidyl Alcohol (UNII: 1QR1QRA9BU) Arachidyl Glucoside (UNII: 6JVW35JOOJ) DOCOSANOL (UNII: 9G1OE216XY) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM OXIDE (UNII: LMI26O6933) Trisodium Ethylenediamine Disuccinate (UNII: YA22H34H9Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59062-1000-6 177 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 07/24/2017 Part 2 of 2 SPF 50 PLUS SUNSCREEN ALL MINERAL ACTIVE INGREDIENTS
titanium dioxide and zinc oxide stickProduct Information Item Code (Source) NDC:59062-1200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 79 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 67 mg in 1 g Inactive Ingredients Ingredient Name Strength ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) YELLOW WAX (UNII: 2ZA36H0S2V) CARNAUBA WAX (UNII: R12CBM0EIZ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) NEOPENTYL GLYCOL DIETHYLHEXANOATE (UNII: U68ZV6W62C) TRIBEHENIN (UNII: 8OC9U7TQZ0) CANDELILLA WAX (UNII: WL0328HX19) PPG-3 BENZYL ETHER MYRISTATE (UNII: 8075L58MKO) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) Stearyl/Octyldodecyl Citrate Crosspolymer (UNII: PN88NW0KPK) JOJOBA OIL (UNII: 724GKU717M) Polyglyceryl-10 Pentaoleate (UNII: BH1TF96DJC) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) JOJOBA OIL, RANDOMIZED (UNII: 7F0EV20QYL) RASPBERRY SEED OIL (UNII: 9S8867952A) CRANBERRY SEED OIL (UNII: 73KDS3BW5E) SUNFLOWER OIL (UNII: 3W1JG795YI) TOMATO SEED OIL (UNII: 7N87T9C06T) NIGELLA SATIVA SEED OIL (UNII: CS4U38E731) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM OXIDE (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59062-1200-1 13 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 06/21/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/01/2017 Labeler - KAS Direct LLC dba BabyGanics (002764605)