Label: PINE AND HONEY SCENT FOAMING HAND SANITIZER- benzalkonium chloride liquid
- NDC Code(s): 74177-041-08, 74177-041-16
- Packager: K7 DESIGN GROUP INC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 26, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
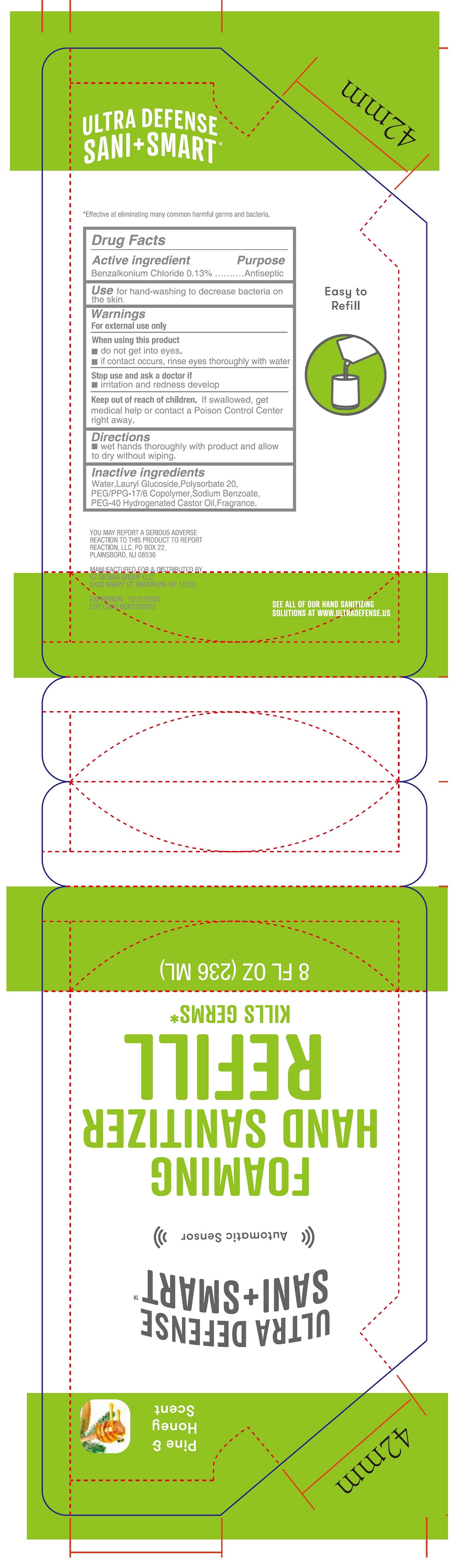
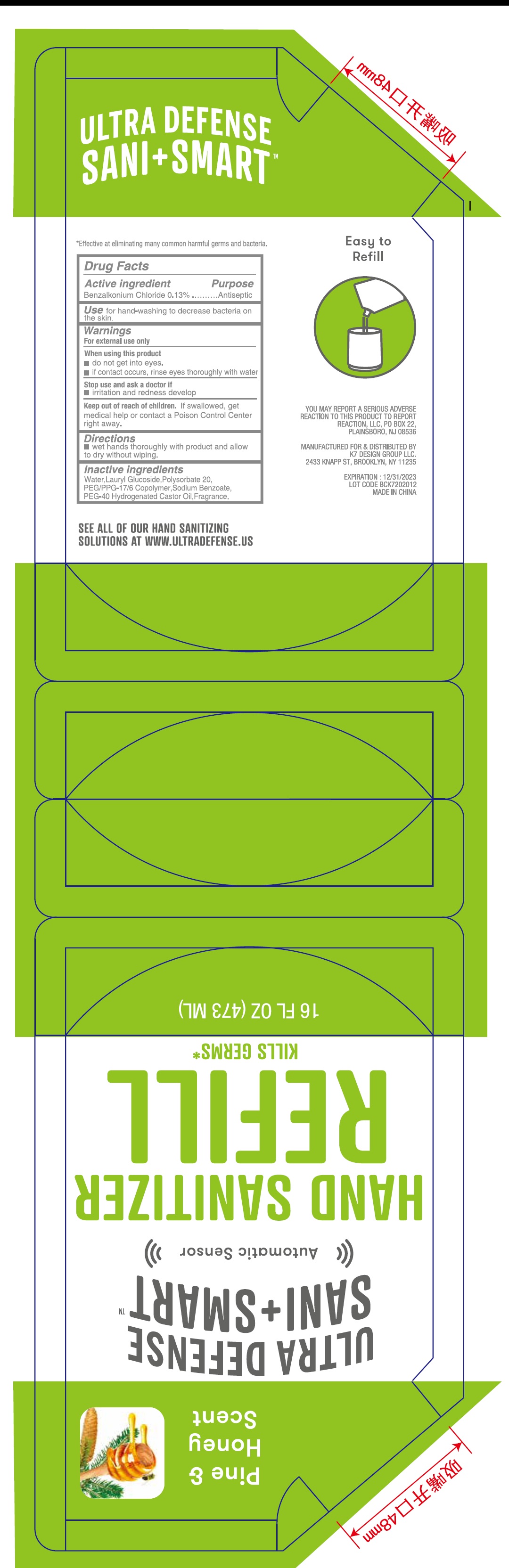
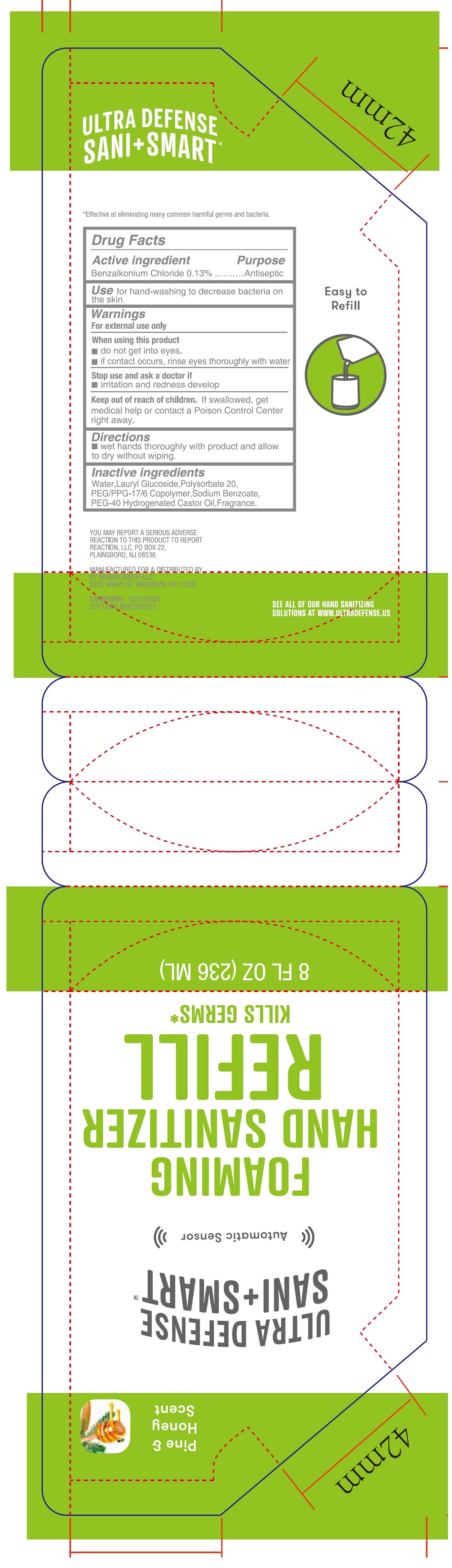
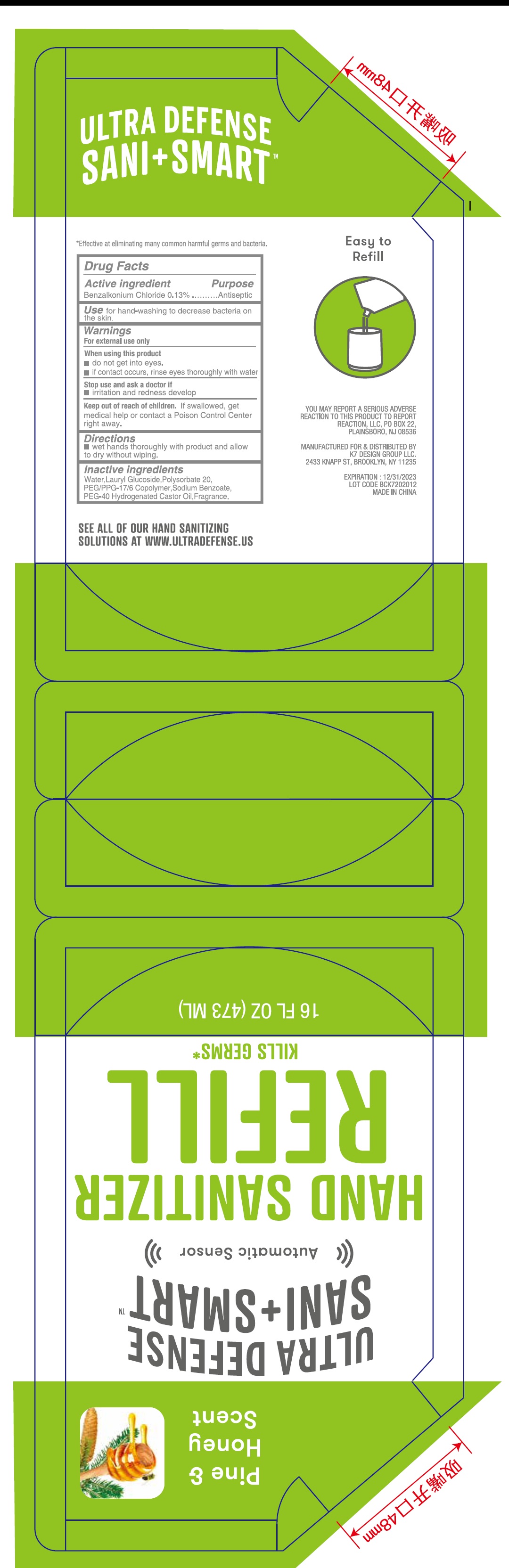
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
- Inactive ingredients
- Package Labeling:236ml
- Package Labeling:473ml
-
INGREDIENTS AND APPEARANCE
PINE AND HONEY SCENT FOAMING HAND SANITIZER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74177-041 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYSORBATE 20 (UNII: 7T1F30V5YH) PEG/PPG-17/6 COPOLYMER (UNII: P5QZM4T259) SODIUM BENZOATE (UNII: OJ245FE5EU) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74177-041-08 236 mL in 1 POUCH; Type 0: Not a Combination Product 01/24/2021 2 NDC:74177-041-16 473 mL in 1 POUCH; Type 0: Not a Combination Product 01/24/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/24/2021 Labeler - K7 DESIGN GROUP INC (080357784) Establishment Name Address ID/FEI Business Operations Bath Concept Cosmetics (Dongguan) Co., Ltd 529623933 manufacture(74177-041)