Label: DIPHENHYDRAMINE HCL solution
- NDC Code(s): 0904-6985-16, 0904-6985-20
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 29, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
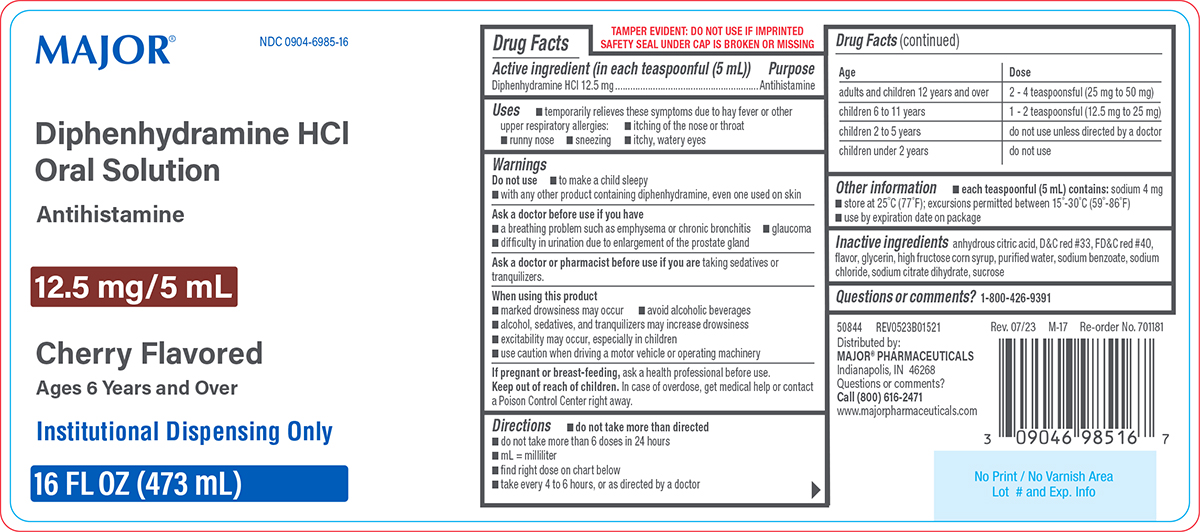
- Active ingredient (in each teaspoonful (5 mL))
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
-
Directions
- do not take more than directed
- do not take more than 6 doses in 24 hours
- mL = milliliter
- find right dose on chart below
- take every 4 to 6 hours, or as directed by a doctor
Age Dose adults and children 12 years and over 2 - 4 teaspoonsful (25 mg to 50 mg) children 6 to 11 years 1 - 2 teaspoonsful (12.5 mg to 25 mg) children 2 to 5 years do not use unless directed by a doctor children under 2 years do not use - Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
MAJOR®
NDC 0904-6985-16
Diphenhydramine HCl
Oral SolutionAntihistamine
12.5 mg/5 mL
Cherry Flavored
Ages 6 Years and Over
Institutional Dispensing only
16 FL OZ (473 mL)
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING50844 REV0523B01521
Distributed by:
MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
Questions or comments?
Call (800) 616-2471
www.majorpharmaceuticals.com
Major 44-015
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HCL
diphenhydramine hcl solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6985 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCROSE (UNII: C151H8M554) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6985-20 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/27/2019 2 NDC:0904-6985-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/27/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/27/2019 Labeler - Major Pharmaceuticals (191427277) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 manufacture(0904-6985) , pack(0904-6985)