Label: ASEPXIA ACNE TREATMENT- salicylic acid cream
- NDC Code(s): 50066-064-01
- Packager: Genomma Lab USA
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 8, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- If irritation or sensitivity develops, stop use and ask a doctor
Sensitivity test for new user
- apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
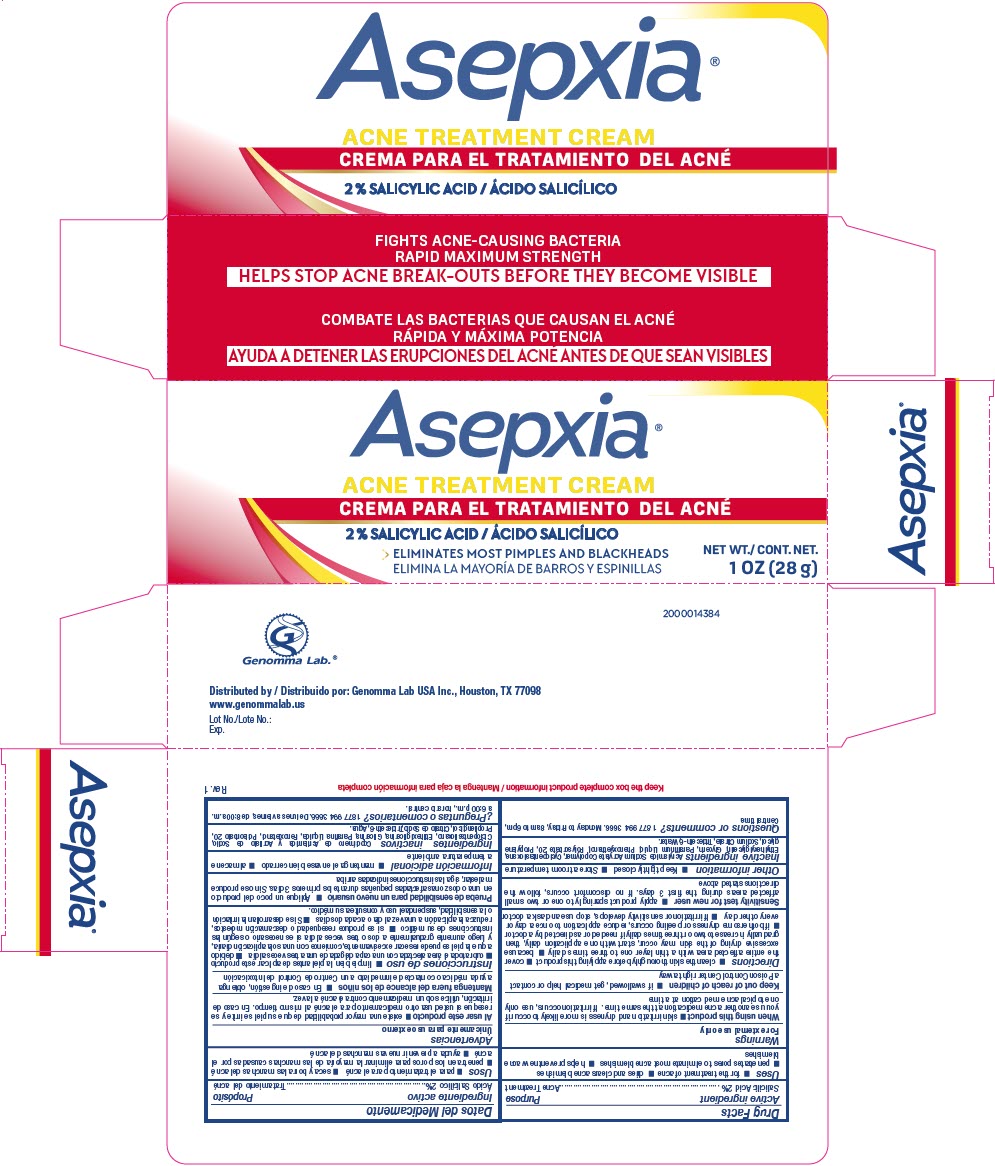
- PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
-
INGREDIENTS AND APPEARANCE
ASEPXIA ACNE TREATMENT
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50066-064 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 20 (UNII: 7T1F30V5YH) PHENOXYETHANOL (UNII: HIE492ZZ3T) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50066-064-01 1 in 1 CARTON 09/05/2024 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/05/2024 Labeler - Genomma Lab USA (832323534)