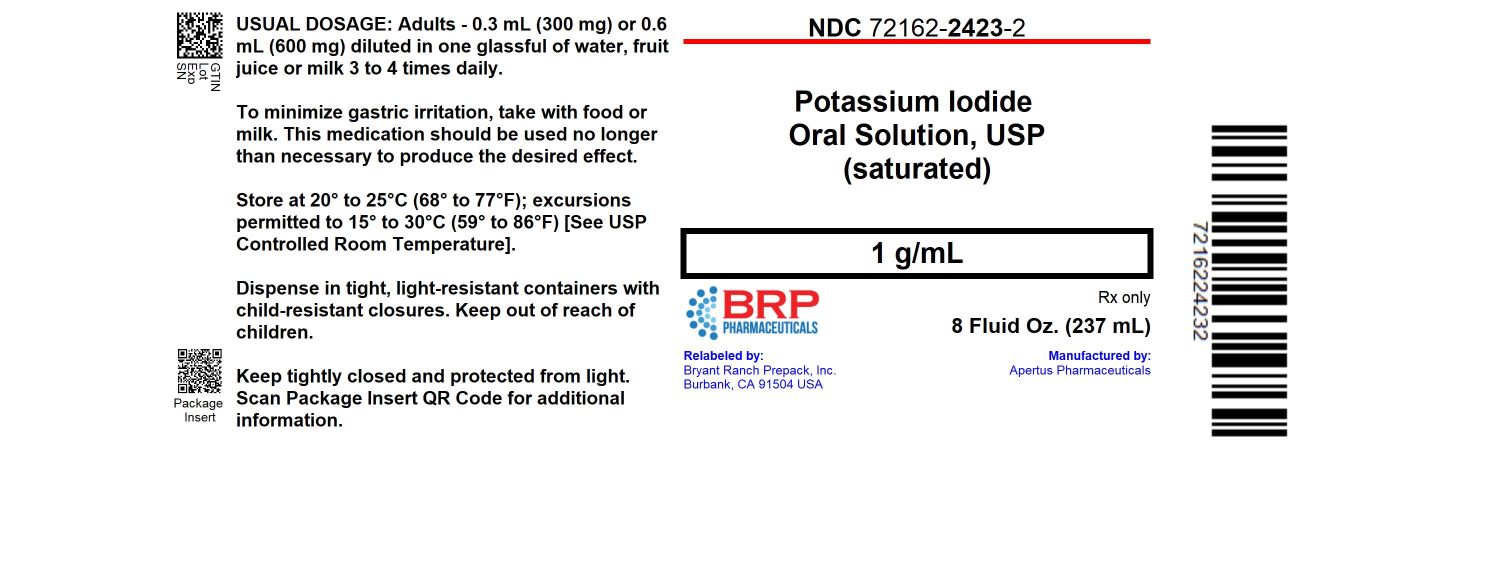
Label: POTASSIUM IODIDE solution
- NDC Code(s): 72162-2423-2, 72162-2423-3
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 75834-280
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
WARNINGS
Potassium iodide can cause fetal harm, abnormal thyroid function, and goiter when administered to a pregnant woman. Because of the possible development of fetal goiter, if the drug is used during pregnancy or if the patient becomes pregnant during therapy, apprise the patient of the potential hazard.
-
PRECAUTIONS
GENERAL
In some patients, prolonged use of iodides can lead to hypothyroidism. Iodides should be used with caution in patients having Addison's disease, cardiac disease, hyperthyroidism, myotonia congenita, tuberculosis, acute bronchitis, or renal function impairment.
DRUG INTERACTIONS
Concurrent use with lithium or antithyroid drugs may potentiate the hypothyroid and goitrogenic effects of these medications. Concurrent use with potassium-containing medications, potassium-sparing diuretics and angiotensin-converting enzyme inhibitors (ACE inhibitors) may result in hyperkalemia and cardiac arrhythmias or cardiac arrest.
-
ADVERSE REACTIONS
The most frequent adverse reactions to potassium iodide are stomach upset, diarrhea, nausea, vomiting, stomach pain, skin rash, and salivary gland swelling or tenderness. Less frequent adverse reactions include gastrointestinal bleeding, confusion, irregular heartbeat, numbness, tingling, pain or weakness in hands or feet, unusual tiredness, weakness or heaviness of legs, fever, and swelling of neck or throat. Thyroid adenoma, goiter, and myxedema are possible side effects.
Iodism or chronic iodine poisoning may occur during prolonged treatment or with the use of high doses. The symptoms of iodism include burning of mouth or throat, severe headache, metallic taste, soreness of teeth and gums, symptoms of head cold, irritation of the eyes with swelling of the eyelids, unusual increase in salivation, acneform skin lesions in the seborrheic areas, and rarely, severe skin eruptions. If symptoms of iodism appear, the drug should be withdrawn and the patient given appropriate supportive therapy.
Hypersensitivity to iodides may occur and may be manifested by angioedema, cutaneous and mucosal hemorrhage, and signs and symptoms resembling serum sickness, such as fever, arthralgia, lymph node enlargement, and eosinophilia.
-
OVERDOSAGE
Acute toxicity from potassium iodide is relatively rare. An occasional individual may show marked sensitivity and the onset of acute poisoning can occur immediately or hours after administration. Angioedema, laryngeal edema and cutaneous hemorrhages may occur.
Iodism or chronic iodine poisoning may occur during prolonged treatment or with the use of high doses. Symptoms of iodism typically disappear soon after discontinuation of the drug. Abundant fluid and salt intake aids in iodide elimination.
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED
Potassium iodide oral solution, USP is supplied in 1 fluid ounce (30 ml) bottles (NDC 72162-2423-03) with a calibrated dropper marked to deliver 0.3 ml (300 mg) and 0.6 ml (600 mg); and 8 fluid ounce (237 ml) bottles (NDC 72162-2423-02). Inactive ingredient: Sodium thiosulfate as a preservative.
NDC: 72162-2423-2: 237 mL in a BOTTLE.
NDC: 72162-2423-3: 30 mL in a BOTTLE, WITH APPLICATOR.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Keep tightly closed and protected from light.
For the 237mL bottle, dispense in tight, light-resistant containers with child-resistant closures. For the 30mL bottle, place the child-resistant cap back on the amber glass bottle after using the clear plastic dropper for dispensing.
Notice: When exposed to cold temperatures, crystallization may occur, but on warming and shaking, the crystals will redissolve. If the solution turns brownish-yellow in color, it should be discarded.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
POTASSIUM IODIDE
potassium iodide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72162-2423(NDC:75834-280) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) POTASSIUM IODIDE 1 g in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM THIOSULFATE (UNII: HX1032V43M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72162-2423-2 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/11/2024 2 NDC:72162-2423-3 30 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 11/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 12/29/2021 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(72162-2423) , RELABEL(72162-2423)