Label: NATURE MINT ANTICAVITY- sodium monofluorophosphate paste, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 76001-001-01, 76001-001-02, 76001-001-03, 76001-001-04, view more76001-001-05, 76001-001-06, 76001-001-07 - Packager: Daxal Cosmetics Private Limited
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 13, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
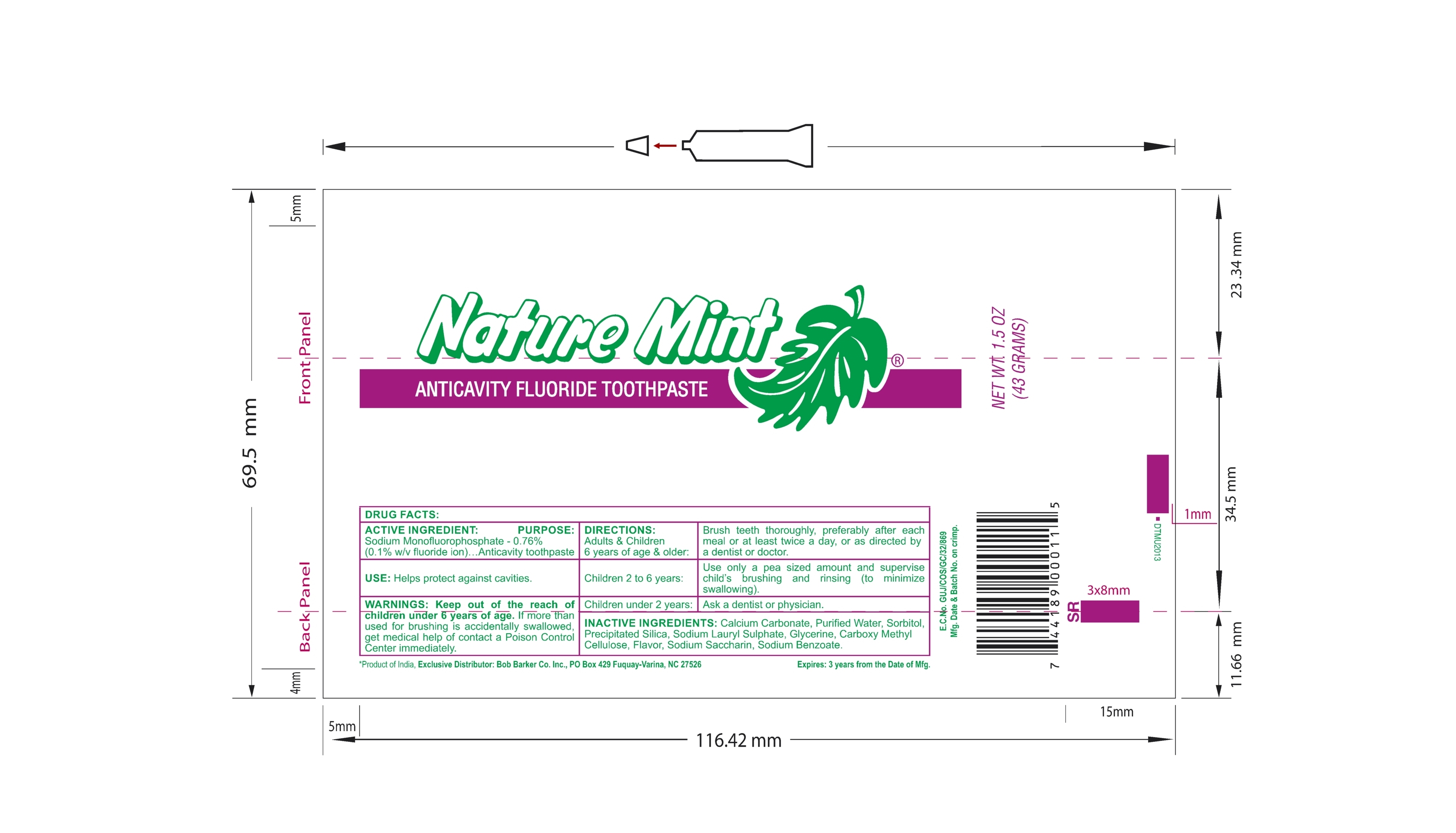
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
-
DIRECTIONS:
Adults & Children 6 years of age & older : Brush teeth throughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
Children 2 to 6 years : Use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing).
Children under 2 years : Ask a dentist or physician.
- PRINCIPAL DISPLAY PANEL - 43 g Tube
-
INGREDIENTS AND APPEARANCE
NATURE MINT ANTICAVITY
sodium monofluorophosphate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76001-001 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.76 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) CALCIUM CARBONATE (UNII: H0G9379FGK) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76001-001-01 7.94 g in 1 PACKET; Type 0: Not a Combination Product 01/01/2018 2 NDC:76001-001-02 17 g in 1 TUBE; Type 0: Not a Combination Product 01/01/2018 3 NDC:76001-001-03 24 g in 1 TUBE; Type 0: Not a Combination Product 01/01/2018 4 NDC:76001-001-04 43 g in 1 TUBE; Type 0: Not a Combination Product 01/01/2018 5 NDC:76001-001-05 78 g in 1 TUBE; Type 0: Not a Combination Product 01/01/2018 6 NDC:76001-001-07 1 in 1 CARTON 01/01/2018 6 181 g in 1 TUBE; Type 0: Not a Combination Product 7 NDC:76001-001-06 1 in 1 CARTON 01/01/2018 7 130 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 01/01/2018 Labeler - Daxal Cosmetics Private Limited (650497790) Establishment Name Address ID/FEI Business Operations Daxal Cosmetics Private Limited 650497790 manufacture(76001-001)