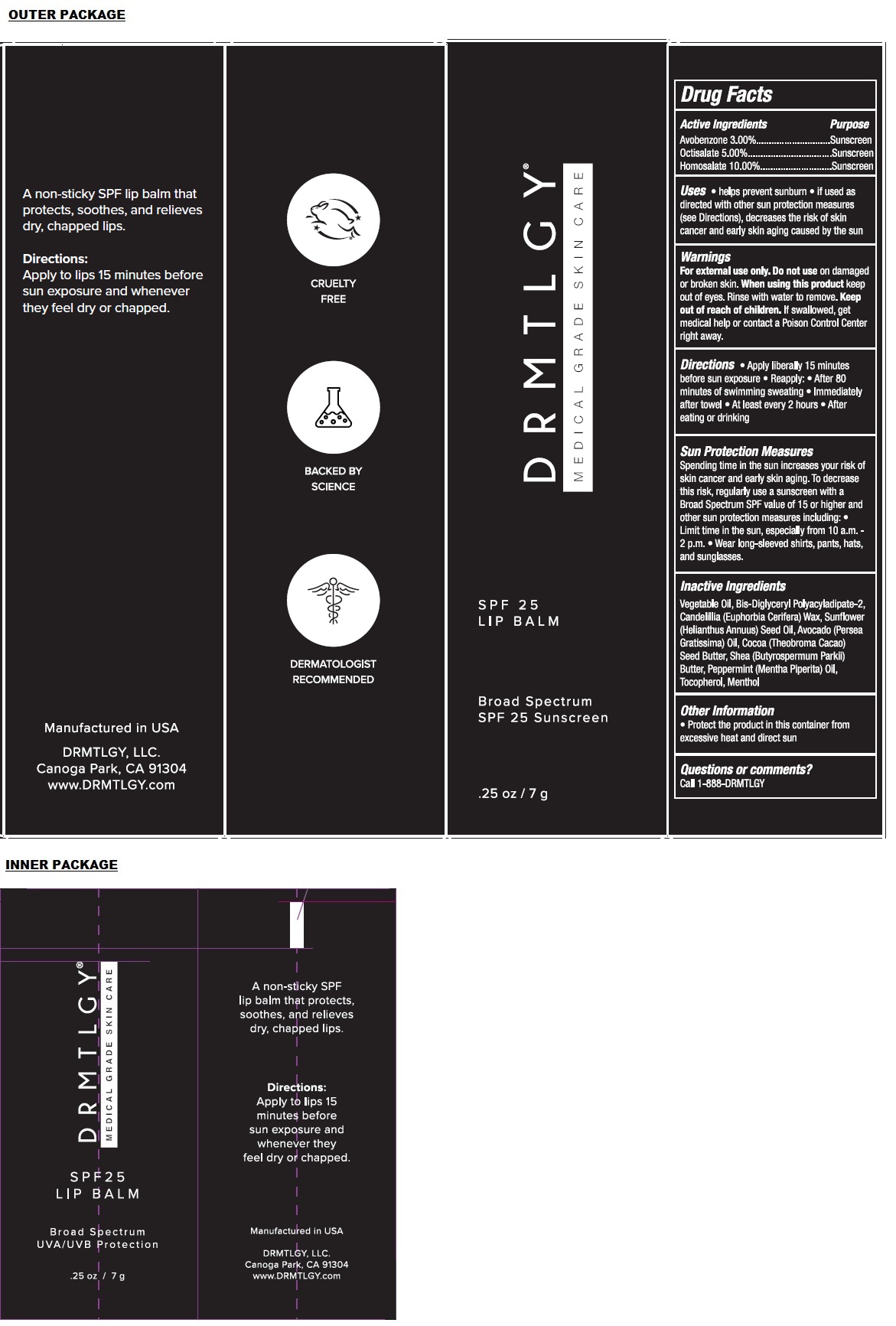
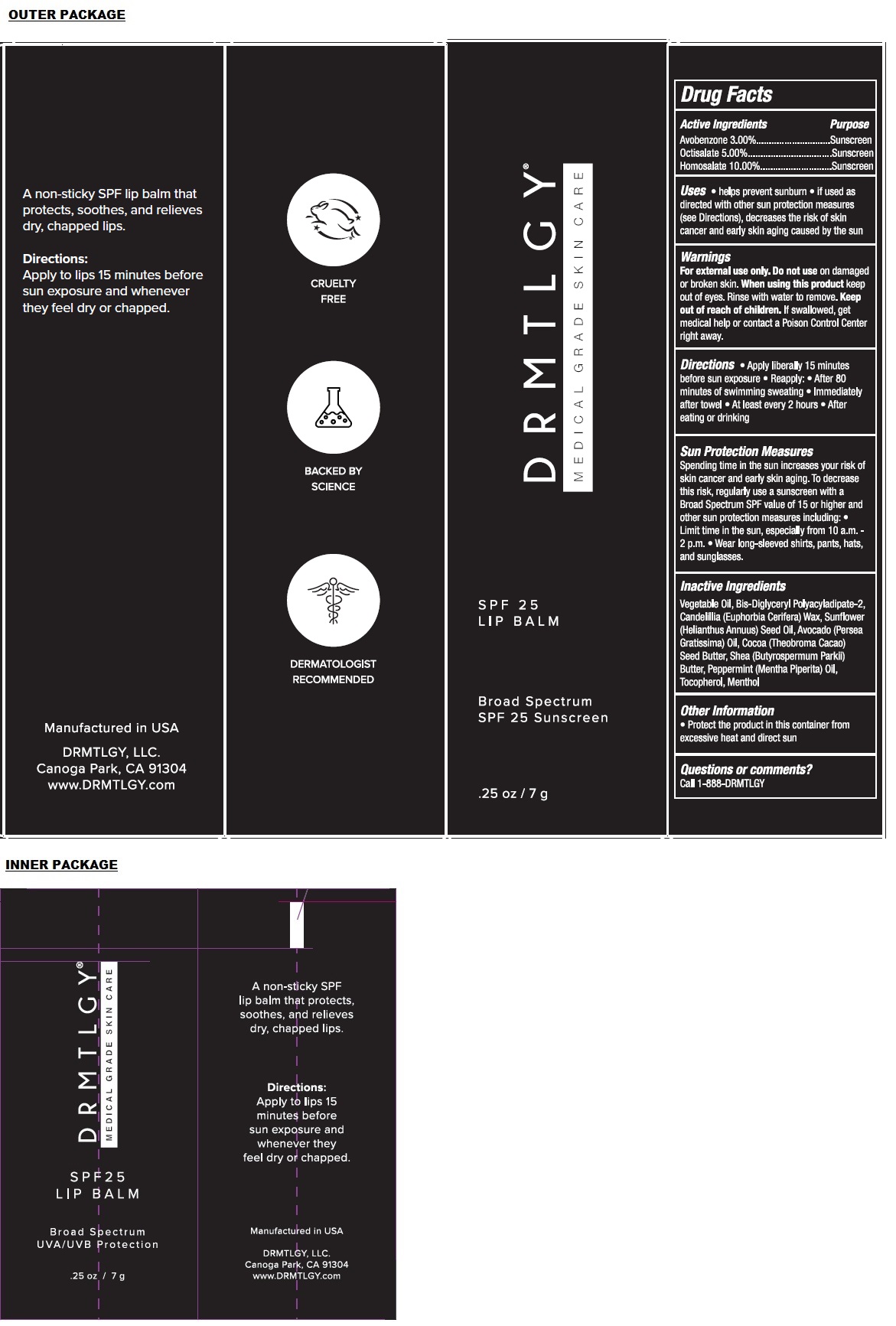
Label: SPF 25 LIP BALM- avobenzone, octisalate, homosalate paste
- NDC Code(s): 83286-002-01
- Packager: Drmtlgy, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 5, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
• Apply liberally 15 minutes before sun exposure • Reapply: • After 80 minutes of swimming sweating • Immediately after towel • At least every 2 hours • After eating or drinking
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. - 2 p.m. • Wear long-sleeved shirts, pants, hats, and sunglasses. - Inactive Ingredients
- Other Information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
SPF 25 LIP BALM
avobenzone, octisalate, homosalate pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83286-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) CANDELILLA WAX (UNII: WL0328HX19) SUNFLOWER OIL (UNII: 3W1JG795YI) AVOCADO OIL (UNII: 6VNO72PFC1) COCOA BUTTER (UNII: 512OYT1CRR) SHEA BUTTER (UNII: K49155WL9Y) PEPPERMINT OIL (UNII: AV092KU4JH) TOCOPHEROL (UNII: R0ZB2556P8) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83286-002-01 1 in 1 CARTON 01/18/2024 1 7 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/18/2024 Labeler - Drmtlgy, LLC (094762235)