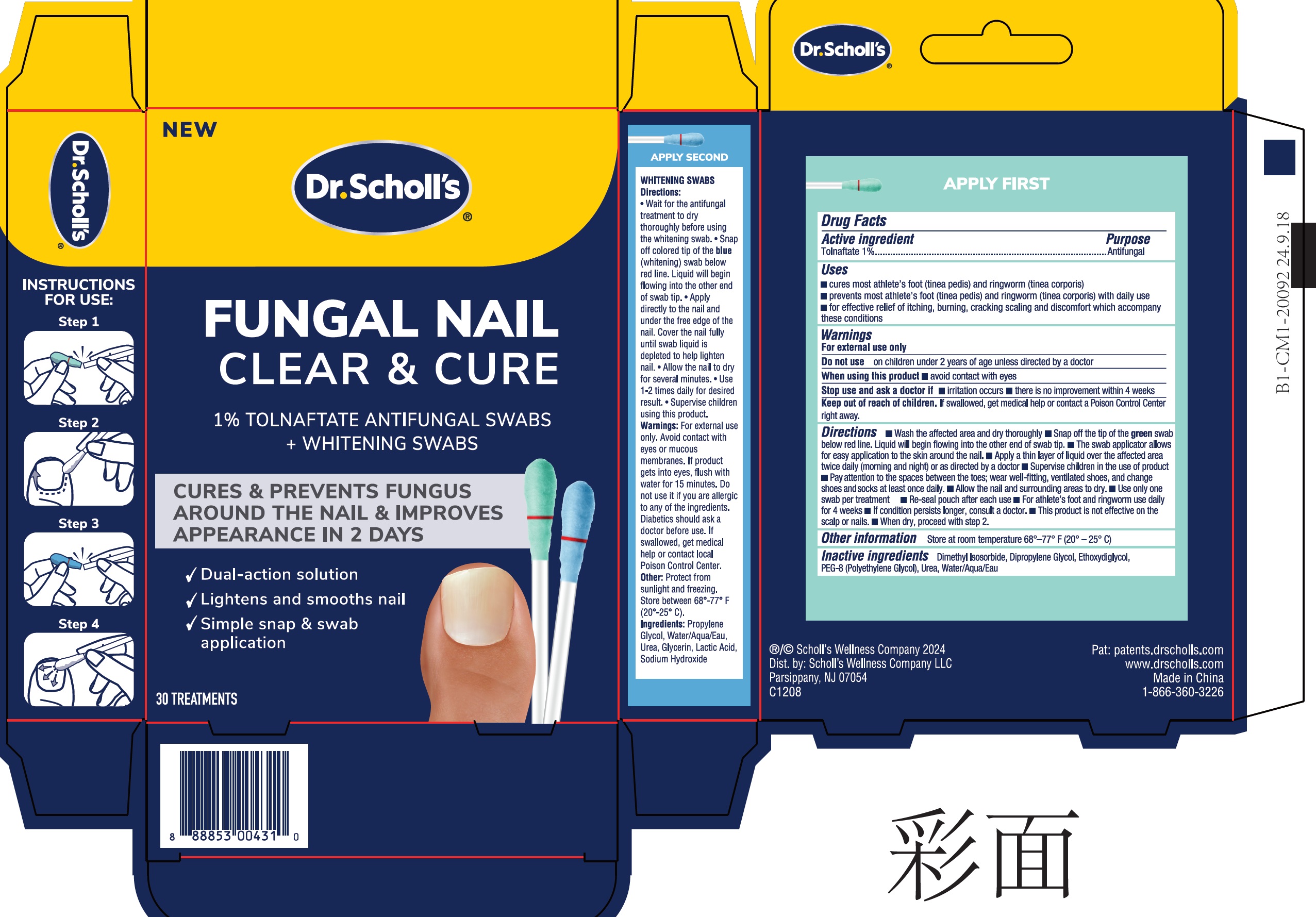
Label: DR SCHOLLS FUNGAL NAIL CLEAR AND CURE TOLNAFTATE ANTIFUNGAL- tolnaftate liquid
- NDC Code(s): 73469-0549-3
- Packager: Scholl's Wellness Company LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 3, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
-
Directions
- Wash the affected area and dry thoroughly
- Snap off the tip of the green swab below red line. Liquid will begin flowing into the other end of swab tip.
- The swab applicator allows for easy application to the skin around the nail.
- Apply a thin layer of liquid over the affected area twice daily (morning and night) or as directed by a doctor
- Supervise children in the use of product
- Pay attention to the spaces between the toes; wear well-fitting,ventilated shoes, and change shoes and socks at least once daily.
- Allow the nail and surrounding areas to dry.
- Use only one swab per treatment
- Re-seal pouch after each use
- For athlete's foot and ringworm use daily for 4 weeks
- If condition persists longer, consult a doctor.
- This product is not effective on the scalp or nails.
- When dry, proceed with step 2.
- Other information
- Inactive ingredients
- Package Labeling: Dr. Scholl's Fungal Nail Clear & Cure 1% Tolnaftate Antifungal Swabs, 30 Count
-
INGREDIENTS AND APPEARANCE
DR SCHOLLS FUNGAL NAIL CLEAR AND CURE TOLNAFTATE ANTIFUNGAL
tolnaftate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73469-0549 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 11 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) DIPROPYLENE GLYCOL (UNII: E107L85C40) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73469-0549-3 1 in 1 CARTON 10/03/2024 1 30 in 1 POUCH 1 0.2 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 10/03/2024 Labeler - Scholl's Wellness Company LLC (117174744)