Label: HEMORRHOID-X TREATMENT PACK kit
- NDC Code(s): 34666-291-01
- Packager: Nartex Laboratorios Homeopaticos SA DE CV
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 12, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Hemorrhoid-x Treatment Pack - Hemorrhoidex Tablets
- Drug Facts
- Active Ingredient (in each tablet)
-
Purpose
Aesculus Hipp 6C HPUS*.........Hemorrhoids, pain after stool, aching, swollen, burning in anus
Collinsonia 6C HPUS*.........Hemorrhoids, itching or burning in anus with swelling
Hamamelis 6C HPUS*.......Hemorrhoids, pulsation in rectum
*The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. "C" is an homeopathic dilution. See www.nartexlabsusa.com formore information.
-
Uses
Temporarily relieves discomforting symptoms such as itching, burning, and swelling associated with hemorrhoids. Product claims and Uses are based on Homeopathic Materia Medica not clinical test. This product and its Uses have not been evaluated by the Food and Drug Administration. This product has not been clinically tested by Nartex Labs USA, Inc.
- Warnings
- ASK DOCTOR
- STOP USE
- WHEN USING
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- Hemorrhoid-x Treatment Pack - Hemorrhoidex ointment
- Drug Facts
- Active Ingredients
-
Purpose
Ratanhia 3X HPUS*....Hemorrhoids, pain after stool, burning in anus
Hamamelis 1X HPUS*.......Hemorrhoids, pulsation in rectum
*The letters HPUS indicate that the components in this product are offically monographed in the Homeopathic Pharmacopoeia of the United States. X is an homeopathic dilution: see www.nartexlabsusa.com for more information.
-
Uses
Temporarily relieves discomforting symptoms such as itching, burning, and swelling associated with hemorrhoids. Product claims and Uses are based on Homeopathic Materia Medica not clinical test. This product and its Uses have not been evaluated by the Food and Drug Administration. This product has not been clinically tested by Nartex Labs USA, Inc. This product has not been shown to relieve symptoms such as itching, burning, and swelling associated with hemorrhoids.
- Warnings
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Apply generously to affected area up to 2 times daily, especially at night, in the morning or after each bowel movement.
- reapply if needed
- Children under 12 years of age, consult a phsyician.
- Other Information
- Inactive Ingredients
- Questions or Comments?
-
Principal Display Panel
TREATMENT PACK
HOMEOPATHIC
HEMORRHOID-X
Temporarily relieves discomforting symptoms such as itching, burning, and swelling associated with
Hemorroids
COMBO pack
There is no scientific evidence that the product works. Product's claims are based only on theories of homeopathy from the 1700's that are not accepted by most modern medical experts.
Contains
1 tube Net. Wt. 1.0oz (28.35g) and 1 box 30 tablets.
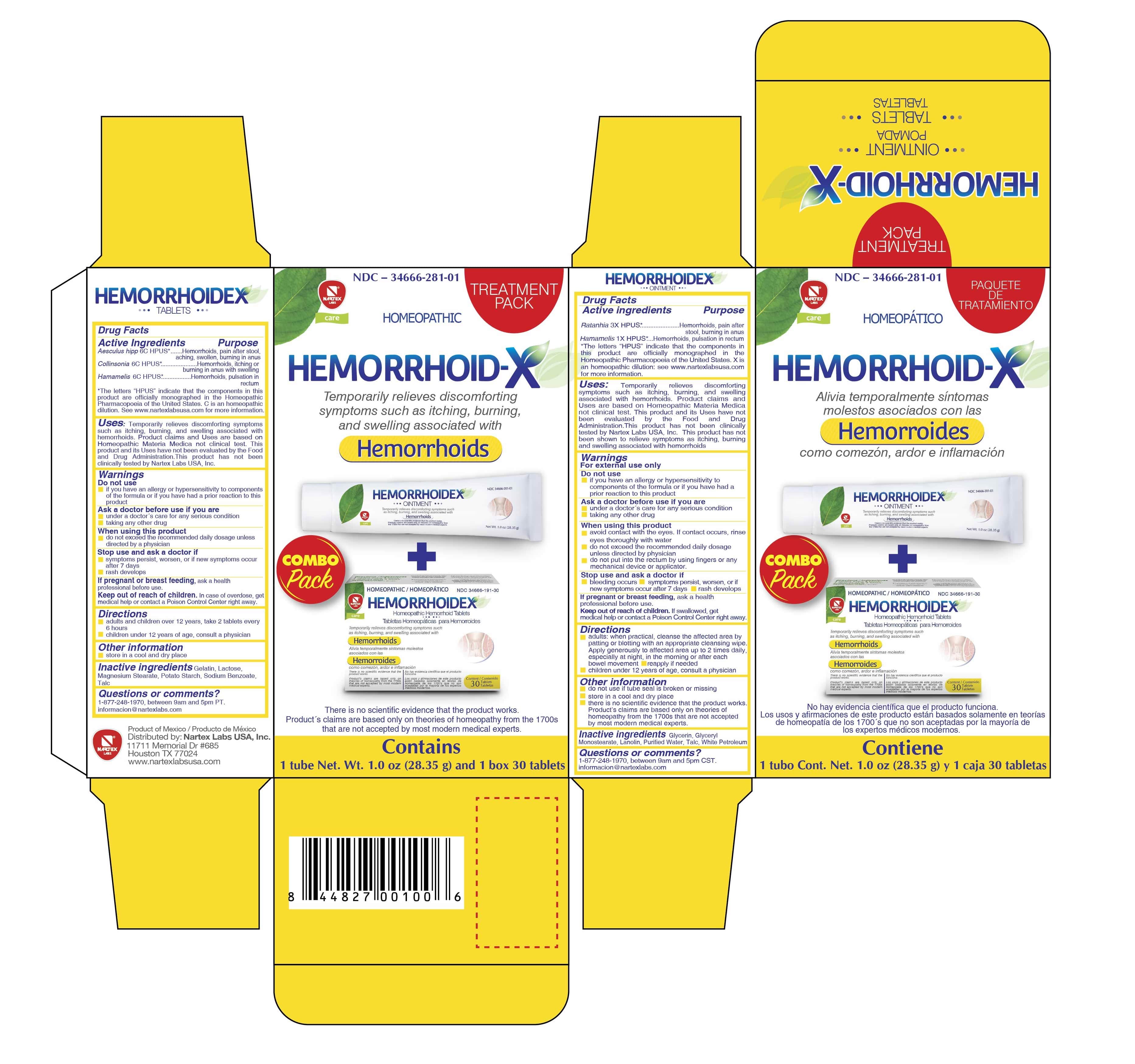
-
INGREDIENTS AND APPEARANCE
HEMORRHOID-X TREATMENT PACK
hemorrhoid-x treatment pack kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:34666-291 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:34666-291-01 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 04/11/2019 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 30 BOX 30 Part 2 2 in 2 Part 1 of 2 HEMORRHOIDEX
hemorrhoidex tabletProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COLLINSONIA CANADENSIS WHOLE (UNII: IOS9HV04K7) (COLLINSONIA CANADENSIS WHOLE - UNII:IOS9HV04K7) COLLINSONIA CANADENSIS WHOLE 6 [hp_C] AESCULUS HIPPOCASTANUM WHOLE (UNII: 2331W47PSX) (AESCULUS HIPPOCASTANUM WHOLE - UNII:2331W47PSX) AESCULUS HIPPOCASTANUM WHOLE 6 [hp_C] HAMAMELIS VIRGINIANA WHOLE (UNII: V663Q8TEFU) (HAMAMELIS VIRGINIANA WHOLE - UNII:V663Q8TEFU) HAMAMELIS VIRGINIANA WHOLE 6 [hp_C] Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) STARCH, POTATO (UNII: 8I089SAH3T) TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (white) Score score with uneven pieces Shape ROUND ((tablet)) Size 10mm Flavor Imprint Code none Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 BOX; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/11/2019 Part 2 of 2 HEMORRHOIDE-X
hemorrhoide-x ointmentProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KRAMERIA LAPPACEA WHOLE (UNII: 97AC37R8BM) (KRAMERIA LAPPACEA WHOLE - UNII:97AC37R8BM) KRAMERIA LAPPACEA WHOLE 3 [hp_X] in 1 g HAMAMELIS VIRGINIANA LEAF (UNII: T07U1161SV) (HAMAMELIS VIRGINIANA LEAF - UNII:T07U1161SV) HAMAMELIS VIRGINIANA LEAF 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) GLYCERIN (UNII: PDC6A3C0OX) TALC (UNII: 7SEV7J4R1U) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LANOLIN (UNII: 7EV65EAW6H) Product Characteristics Color white Score Shape FREEFORM Size Flavor Imprint Code Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/11/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/11/2019 Labeler - Nartex Laboratorios Homeopaticos SA DE CV (589914576) Registrant - Nartex Laboratorios Homeopaticos SA DE CV (589914576) Establishment Name Address ID/FEI Business Operations Nartex Laboratorios Homeopaticos SA DE CV 589914576 manufacture(34666-291)

