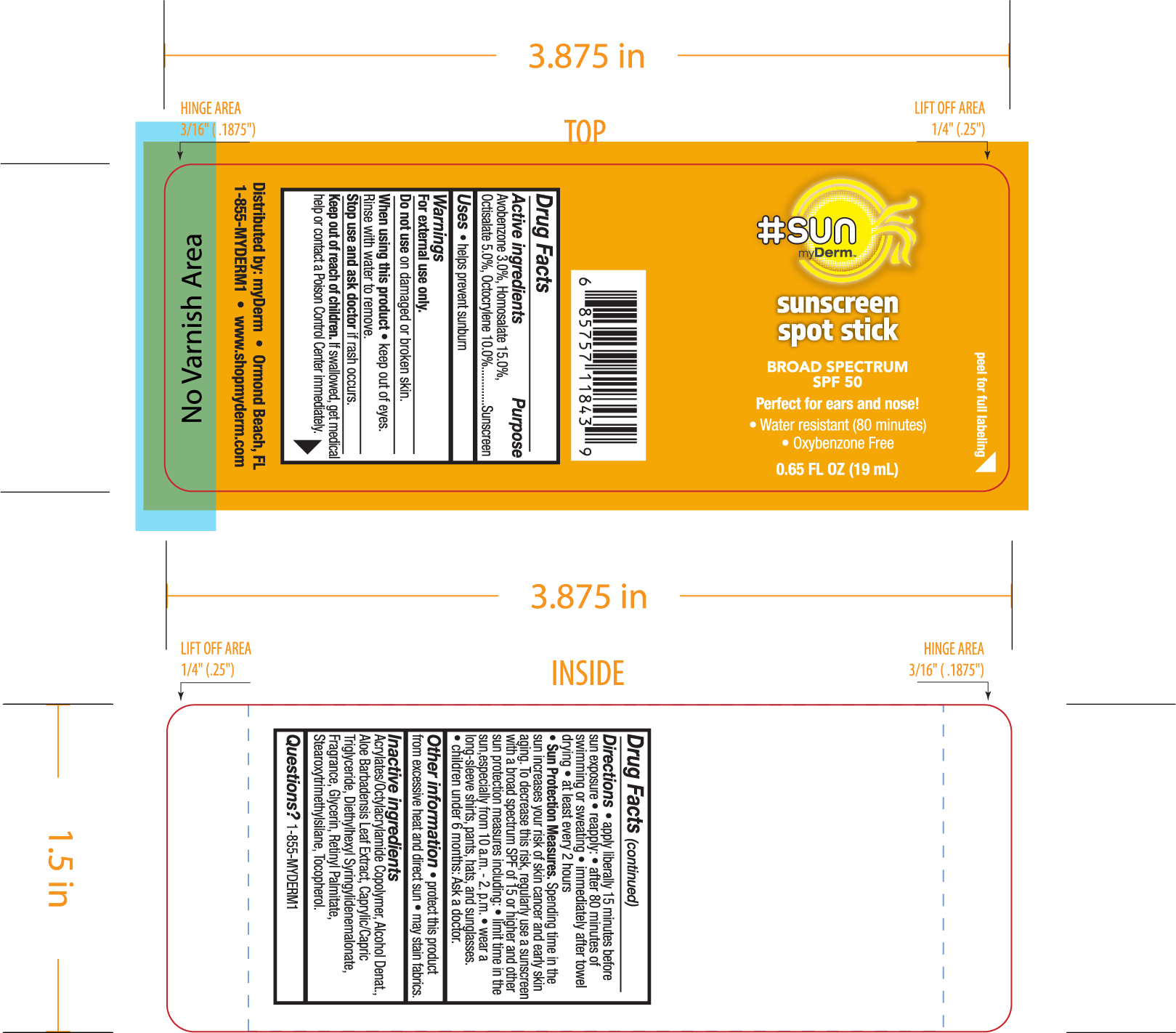
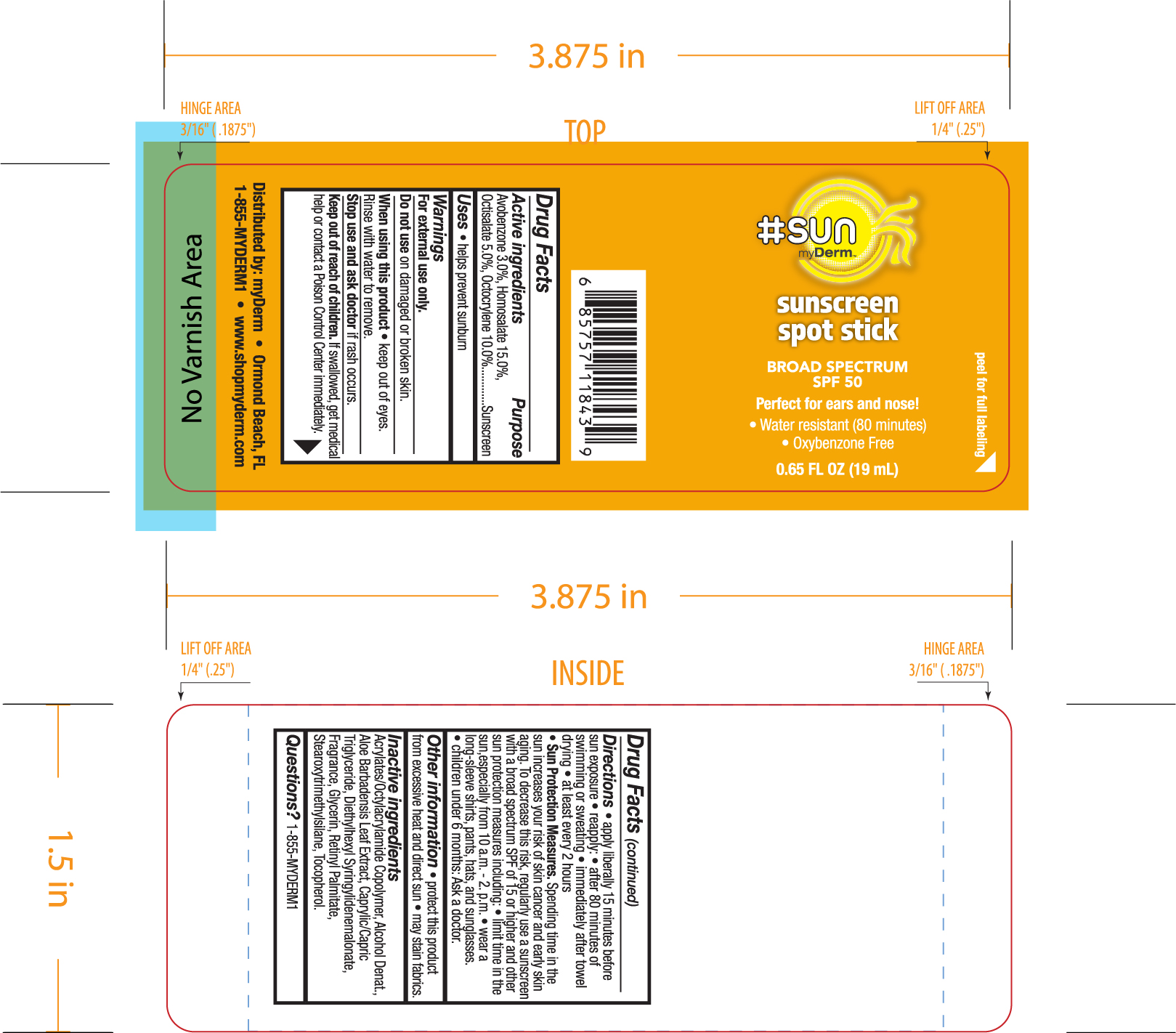
Label: HASHTAGSUN SPF 50 SUNSCREEN DABBER- avobenzone, humosalate, octisalate, octocrylene sponge
-
Contains inactivated NDC Code(s)
NDC Code(s): 72667-017-01 - Packager: Inspec Solutions
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 14, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WHEN USING
- PURPOSE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Behenyl Alcohol
BHT
Butyloctyl Salicylate
Caprylyl Methicone Dimethicone
Dimethyl Capramide
Disodium EDTA
Ethylhexyl Stearate
Ethylhexylglycerin
Fragrance
Glyceryl Stearate Hydrated Silica
PEG-100 Stearate
Phenoxyethanol
Polyester-8
Sodium Polyacrylate
Styrene/Acrylates Copolymer
Trideceth-6
Trimethylsiloxysilicate
VP/Hexadecene Copolymer
Water
Xanthan Gum
- WHEN USING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HASHTAGSUN SPF 50 SUNSCREEN DABBER
avobenzone, humosalate, octisalate, octocrylene spongeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72667-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 15 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength ACRYLATE/ISOBUTYL METHACRYLATE/N-TERT-OCTYLACRYLAMIDE COPOLYMER (75000 MW) (UNII: JU3XHR8VWK) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) ALOE VERA LEAF (UNII: ZY81Z83H0X) BEHENYL BEHENATE (UNII: K8NU647RJ0) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) GLYCERIN (UNII: PDC6A3C0OX) TOCOPHEROL (UNII: R0ZB2556P8) STEAROXYTRIMETHYLSILANE (UNII: 9862TW94B2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72667-017-01 19 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/29/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/29/2021 Labeler - Inspec Solutions (081030372) Establishment Name Address ID/FEI Business Operations Inspec Solutions 081030372 manufacture(72667-017)