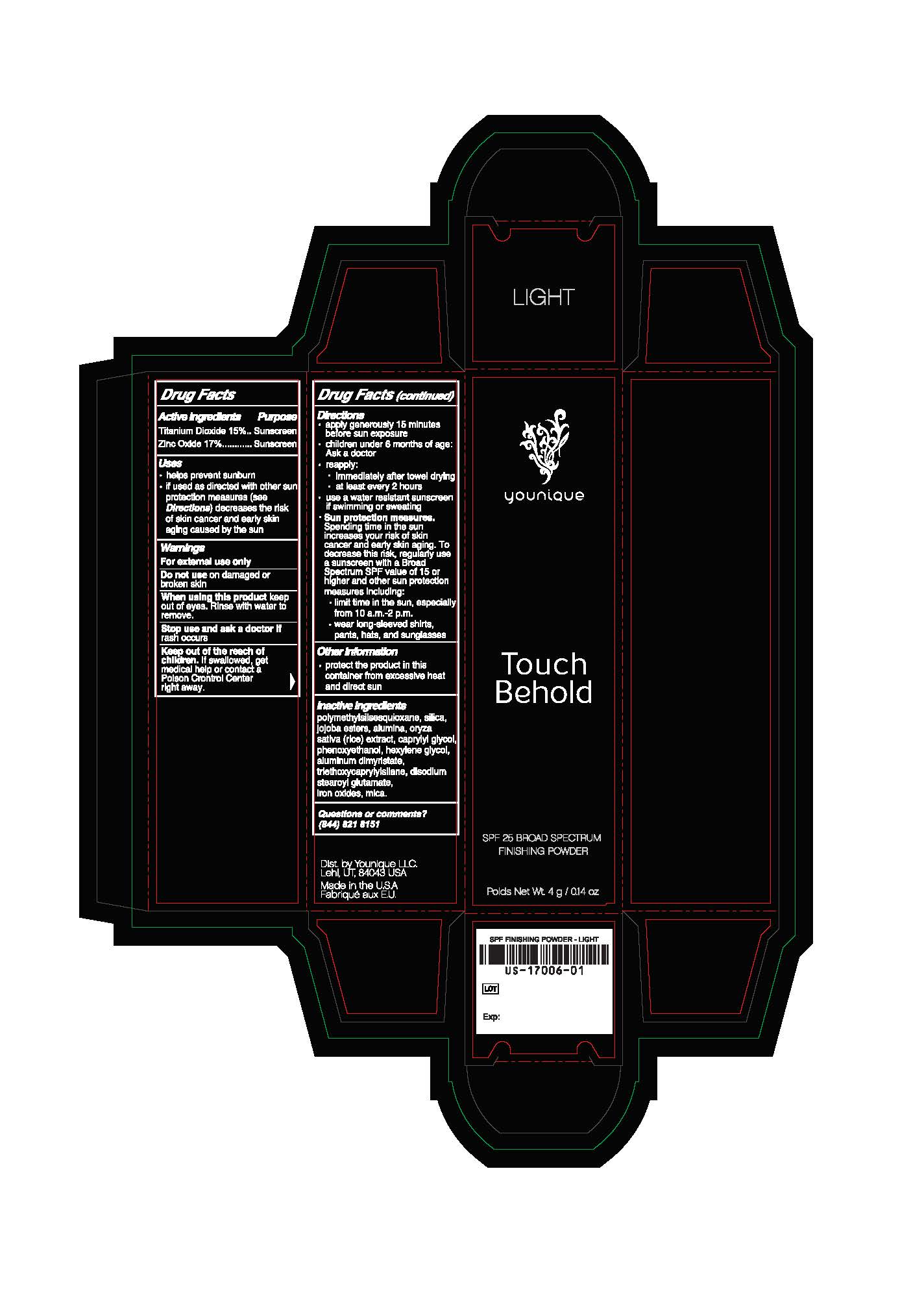
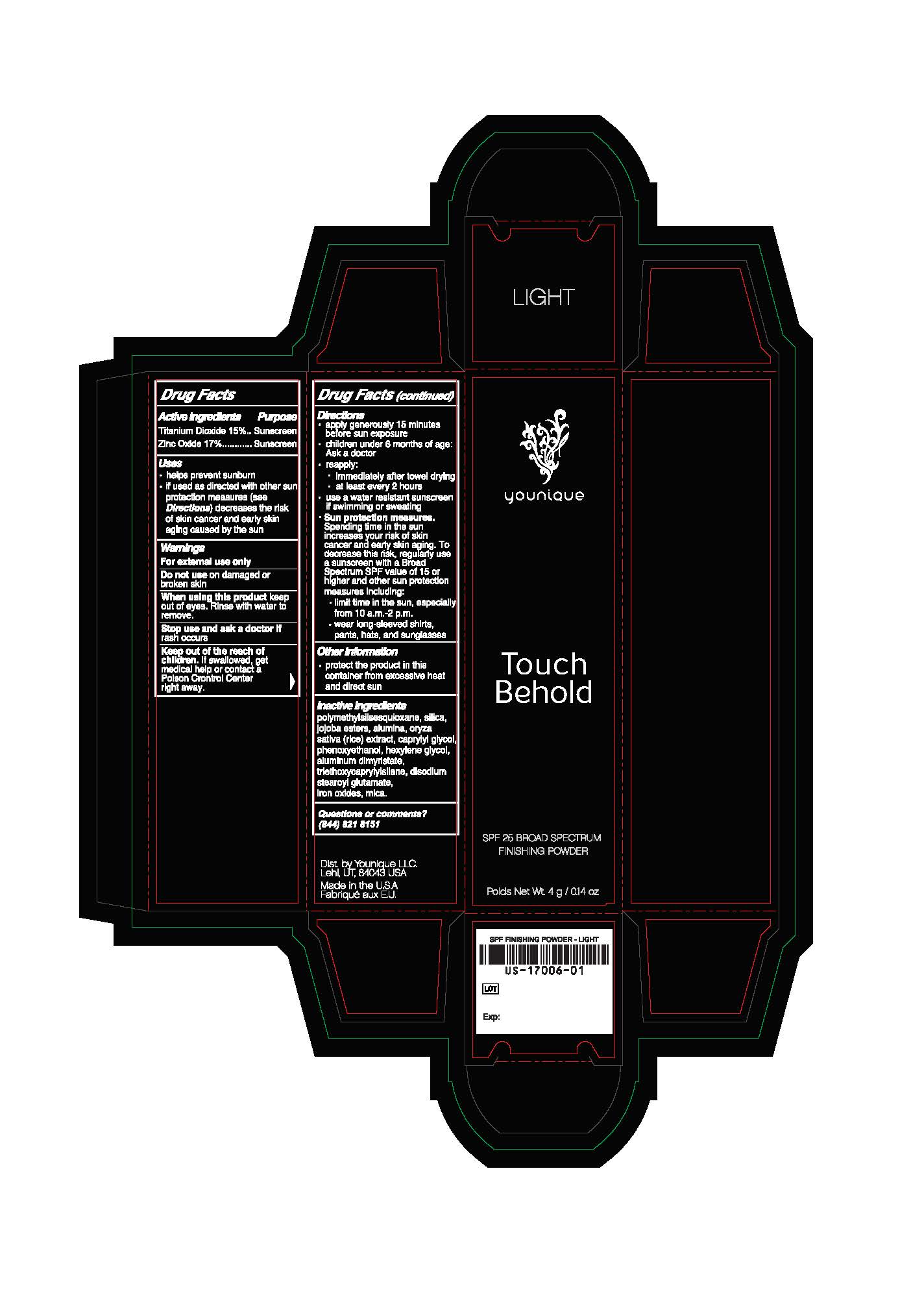
Label: SPF 25 FINISHING POWDER- zinc oxide, titanium dioxide powder
- NHRIC Code(s): 71182-1706-4
- Packager: Younique LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 12, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Directions
- Inactive ingredients
- Directions & Uses
- Warnings
- Purpose
- Active ingredients
- Warnings
- Package.Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SPF 25 FINISHING POWDER
face powders powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NHRIC:71182-1706 Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ZINC OXIDE (UNII: SOI2LOH54Z) 0.175 g in 1 g INGR POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) INGR HYDRATED SILICA (UNII: Y6O7T4G8P9) INGR ALUMINUM OXIDE (UNII: LMI26O6933) INGR RICE OIL (UNII: ACU8G2V809) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) INGR TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) INGR DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) INGR MICA (UNII: V8A1AW0880) INGR HYDROLYZED JOJOBA ESTERS (POTASSIUM SALTS) (UNII: CH428W5O62) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR BROWN IRON OXIDE (UNII: 1N032N7MFO) INGR TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.15 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71182-1706-4 1 in 1 CARTON 10/12/2018 1 4 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 10/12/2018 Labeler - Younique LLC (034555625) Registrant - Younique LLC (034555625) Establishment Name Address ID/FEI Business Operations Oxygen Development, LLC 137098492 manufacture(71182-1706)