Label: LOFEXIDINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 66993-345-37, 66993-345-76
- Packager: Prasco Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated August 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LOFEXIDINE TABLETS safely and effectively. See full prescribing information for Lofexidine tablets. Lofexidine tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGELofexidine tablets are indicated for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The usual Lofexidine tablet starting dosage is three 0.18 mg tablets taken orally 4 times daily during the period of peak withdrawal symptoms (generally the first 5 to 7 ...
-
3 DOSAGE FORMS AND STRENGTHSLofexidine tablets are available as round, peach-colored, film-coated tablets, imprinted with "LFX" on one side and "18" on the other side. Each tablet contains 0.18 mg lofexidine (equivalent to ...
-
4 CONTRAINDICATIONS(What is this?)None.
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Hypotension, Bradycardia, and Syncope - Lofexidine tablets can cause a decrease in blood pressure, a decrease in pulse, and syncope [see Adverse Reactions (6.1), Clinical Pharmacology ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in labeling: Hypotension, Bradycardia, and Syncope [see Warnings and Precautions (5.1)] QT Prolongation [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Methadone - Lofexidine tablets and methadone both prolong the QT interval. ECG monitoring is recommended in patients receiving methadone and Lofexidine tablets concomitantly [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The safety of Lofexidine tablets in pregnant women has not been established. In animal reproduction studies, oral administration of lofexidine during ...
-
10 OVERDOSAGEOverdose with Lofexidine tablets may manifest as hypotension, bradycardia, and sedation. In the event of acute overdose, perform gastric lavage where appropriate. Dialysis will not remove a ...
-
11 DESCRIPTIONLofexidine tablets contain lofexidine, a central alpha-2 adrenergic agonist, as the hydrochloride salt. Lofexidine hydrochloride is chemically designated as 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lofexidine is a central alpha-2 adrenergic agonist that binds to receptors on adrenergic neurons. This reduces the release of norepinephrine and decreases sympathetic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No adequate long-term animal studies have been completed to evaluate the carcinogenic potential of ...
-
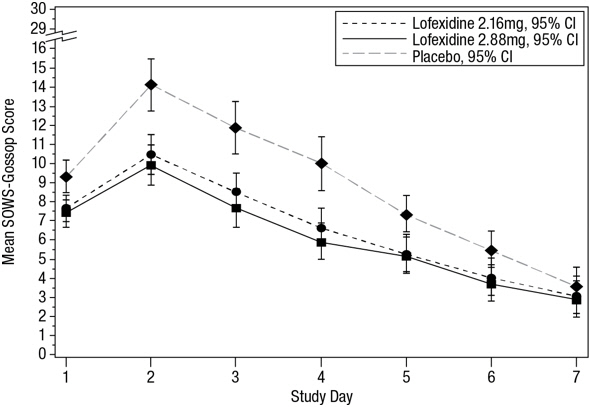
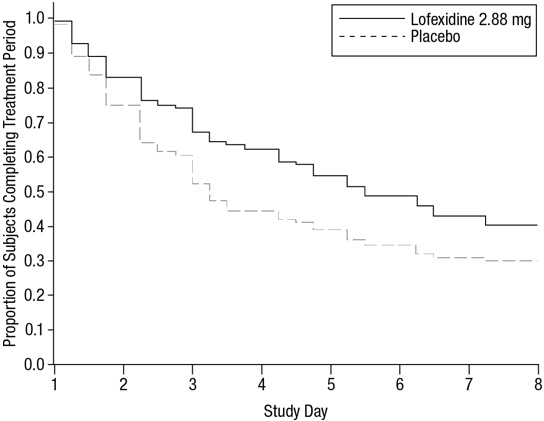
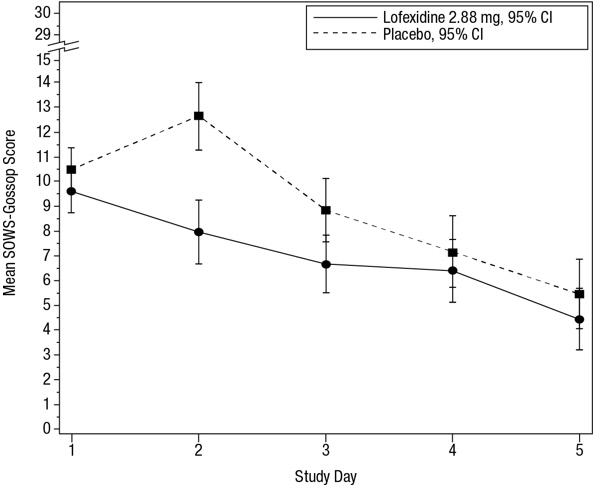
14 CLINICAL STUDIESTwo randomized, double-blind, placebo-controlled trials supported the efficacy of lofexidine. Study 1, NCT01863186 - Study 1 was a 2-part efficacy, safety, and dose-response study conducted in ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Available as 0.18 mg round, convex-shaped, peach colored, film-coated tablets, imprinted with "LFX" on one side and "18" on the other side; approximately 7 mm in ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information). Lofexidine tablets may mitigate, but not completely prevent, the symptoms associated with opioid withdrawal ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Prasco Laboratories - Mason, OH 45040 USA - Under Sublicense from USWM, LLC. 640113 Rev. 08/2023
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 08/2023 - PATIENT INFORMATION - LOFEXIDINE TABLETS - (loe fex' I ...
-
PRINCIPAL DISPLAY PANEL - 0.18 mg Tablet Carton 36 count(What is this?)NDC 66993-345-37 - 36 Tablets - Lofexidine Tablets - 0.18 mg - Store and dispense - in original container. Protect from heat and moisture. Do not remove desiccants. Keep the bottle tightly ...
-
PRINCIPAL DISPLAY PANEL - 0.18 mg Tablet Carton 96 countNDC 66993-345-76 - 96 Tablets - Lofexidine Tablets - 0.18 mg - Store and dispense - in original container. Protect from heat and moisture. Do not remove desiccants. Keep the bottle tightly ...
-
INGREDIENTS AND APPEARANCEProduct Information