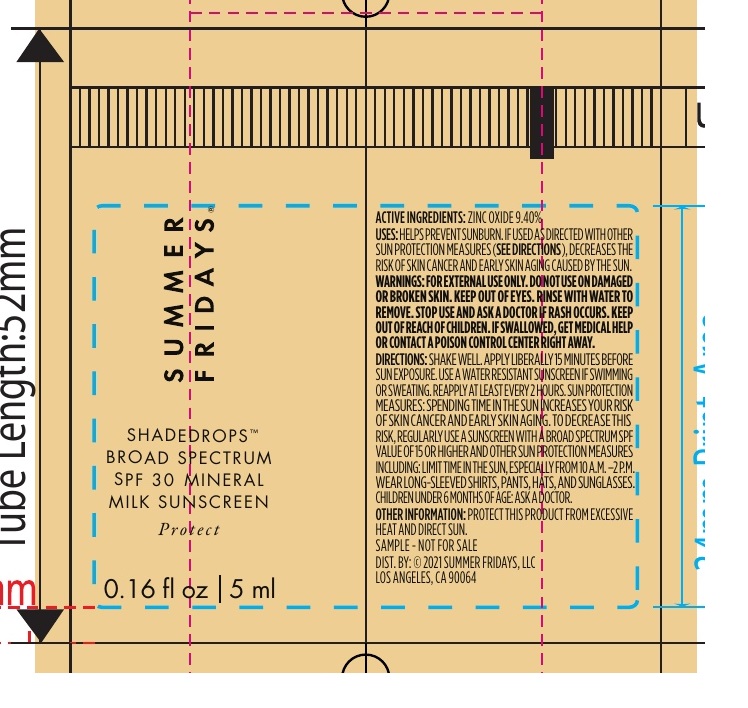
Label: SUMMER FRIDAYS SHADEDROPS BROAD SPECTRUM SPF 30 MINERAL MILK SUNSCREEN- zinc oxide cream
- NDC Code(s): 81678-000-01, 81678-000-02
- Packager: Summer Fridays LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 26, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
• Shake well • Apply liberally 15 minutes before sun exposure • Use a water resistant sunscreen if swimming or sweating • Reapply at least every 2 hours • Children under 6 months of age: Ask a doctor • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. – 2 p.m. • Wear long-sleeved shirts, pants, hats, and sunglasses
Sun Protection Measures. - Other Information
-
Inactive Ingredients
Water, C12-15 Alkyl Benzoate, Sorbitan Oleate Decylglucoside Crosspolymer, Argania Spinosa Kernel Oil, Tocopheryl Acetate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Glycerin, Caramel, Polyester-5, Squalane, Butyrospermum Parkii (Shea) Butter Extract, Bisabolol, Phospholipids, Tocopherol, Ethyl Ferulate, Urea, Ethylhexylglycerin, Gluconolactone, Triethoxycaprylylsilane, Dimethicone, Sodium Chloride, Benzyl Alcohol, Sodium Benzoate, Sorbic Acid, Chlorphenesin, Phenoxyethanol.
- Package Labeling:81678-000-01
- Package Labeling:81678-000-02
-
INGREDIENTS AND APPEARANCE
SUMMER FRIDAYS SHADEDROPS BROAD SPECTRUM SPF 30 MINERAL MILK SUNSCREEN
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81678-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 94 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ARGAN OIL (UNII: 4V59G5UW9X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) GLYCERIN (UNII: PDC6A3C0OX) CARAMEL (UNII: T9D99G2B1R) SQUALANE (UNII: GW89575KF9) SHEA BUTTER (UNII: K49155WL9Y) LEVOMENOL (UNII: 24WE03BX2T) TOCOPHEROL (UNII: R0ZB2556P8) ETHYL FERULATE (UNII: 5B8915UELW) UREA (UNII: 8W8T17847W) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCONOLACTONE (UNII: WQ29KQ9POT) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBIC ACID (UNII: X045WJ989B) CHLORPHENESIN (UNII: I670DAL4SZ) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81678-000-01 1 in 1 CARTON 05/01/2022 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:81678-000-02 1 in 1 CARTON 05/01/2022 2 5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/01/2022 Labeler - Summer Fridays LLC (118620216)