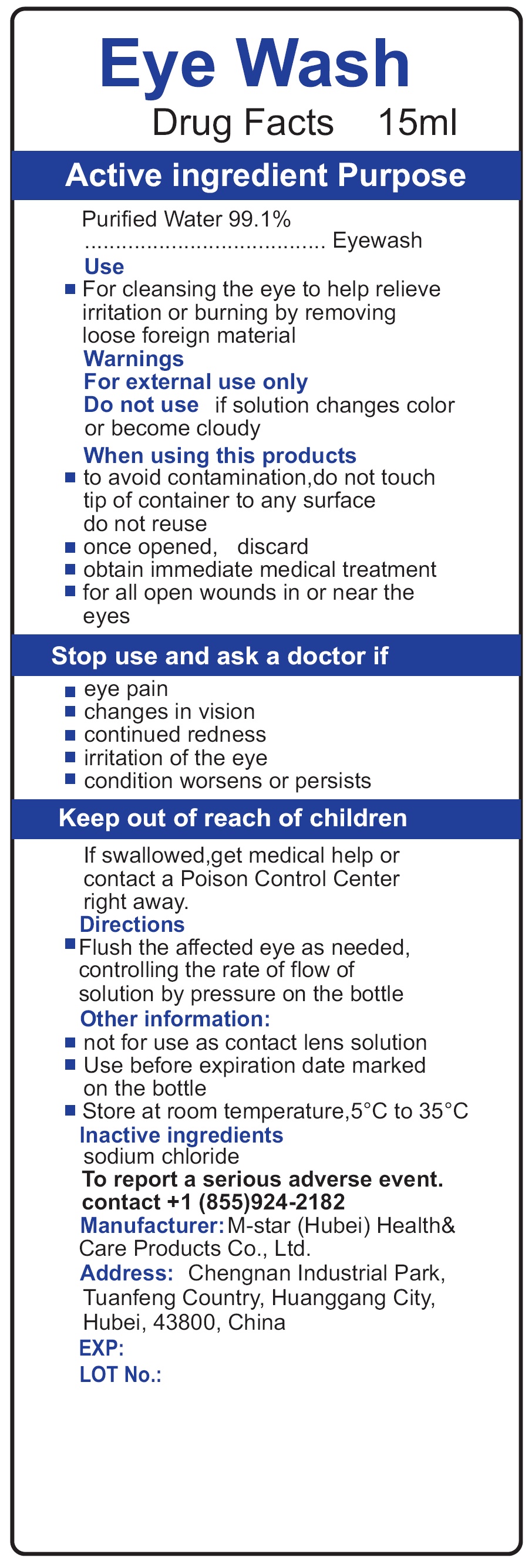
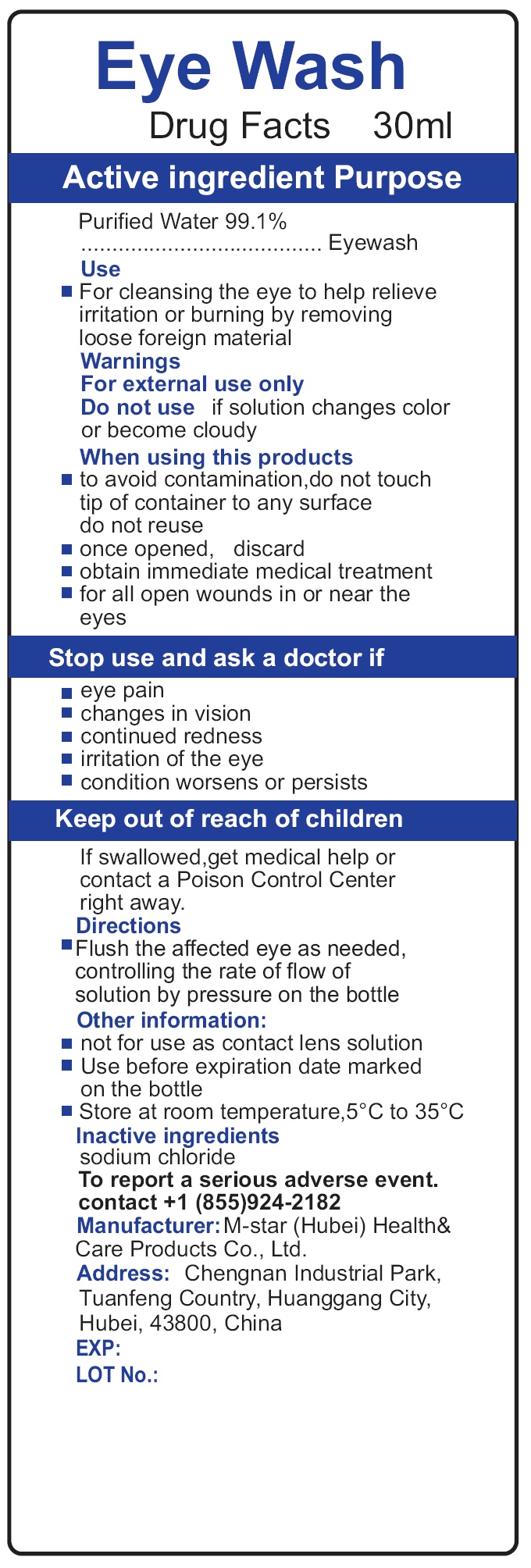
Label: EYE WASH- water liquid
- NDC Code(s): 84219-007-15, 84219-007-30
- Packager: M-star (Hubei) Health&Care Products Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 1, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
-
Warnings
For external use only.
when using this products
- to avoid contamination,do not touch tip of container to any surface do not reuse
- once opened, discard
- obtain immediate medical treatment
- for all open wounds in or near the eyes
- Directions
- Other information
- Inactive ingredients
- To report a serious adverse event.
- Package Labelling:
- Package Labelling:
-
INGREDIENTS AND APPEARANCE
EYE WASH
water liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84219-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 99.1 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84219-007-15 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/15/2024 2 NDC:84219-007-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 05/15/2024 Labeler - M-star (Hubei) Health&Care Products Co., Ltd. (409376382)