Label: ALMAY CLEAR COMPLEXION LIQUID MAKEUP- salicylic acid liquid
- NDC Code(s): 0311-0720-01
- Packager: Almay
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 19, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
-
WHEN USING
Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If occurs only use one topical acne medication at the same time.
Avoid contact with eyes. If contact occurs, flush throughly with water.
Sensitivity test for a new user:
Apply product sparingly to one or two small affected areas during the first three days. If no discomfort occurs, follow the directions above.
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Directions:
Clean skin througly before applying this product. Cover entire affected area with a thin layer one to three times daily. Because excessive drying of the skin may occur , start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
INDICATIONS & USAGE
Acne treatment
Directions:
Clean skin througly before applying this product. Cover entire affected area with a thin layer one to three times daily. Because excessive drying of the skin may occur , start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
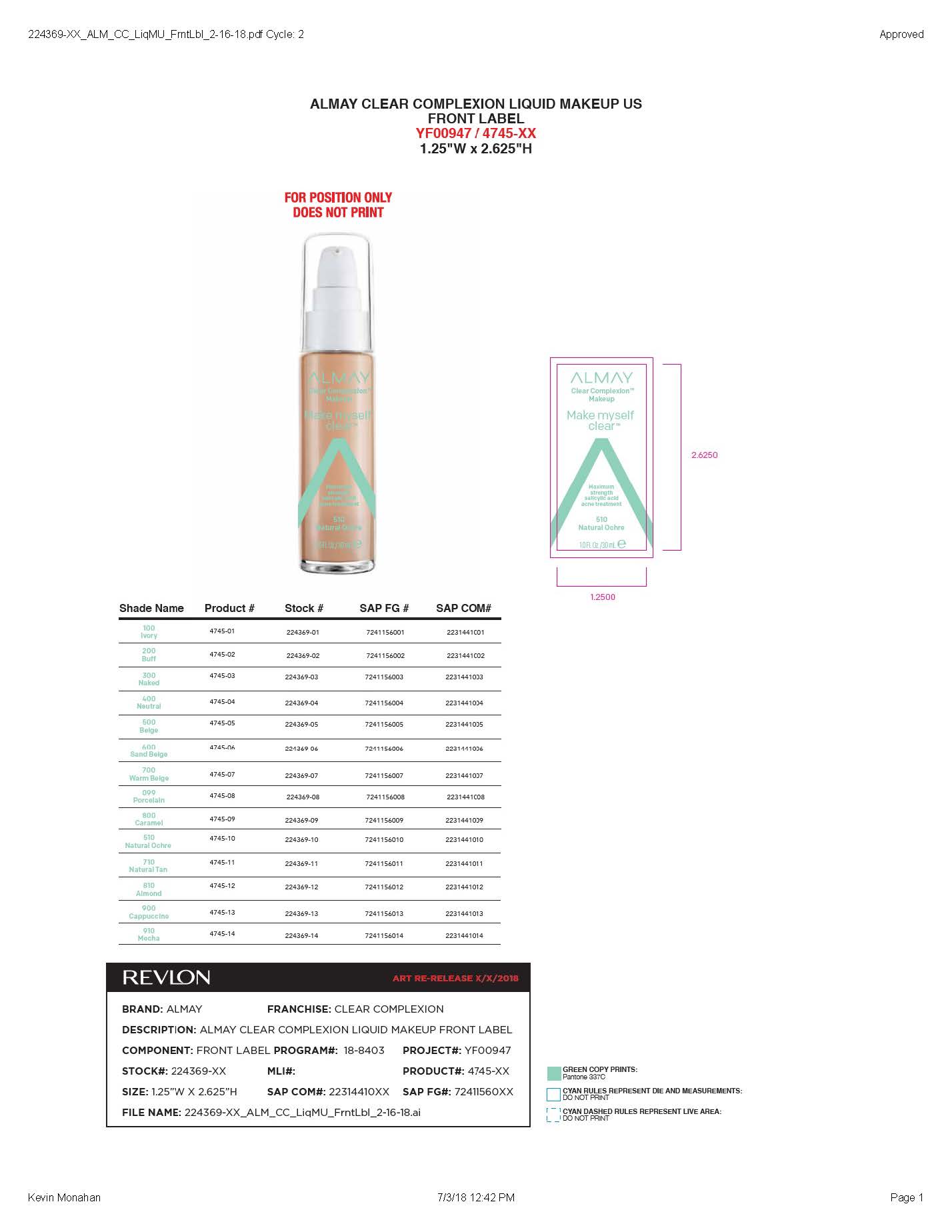
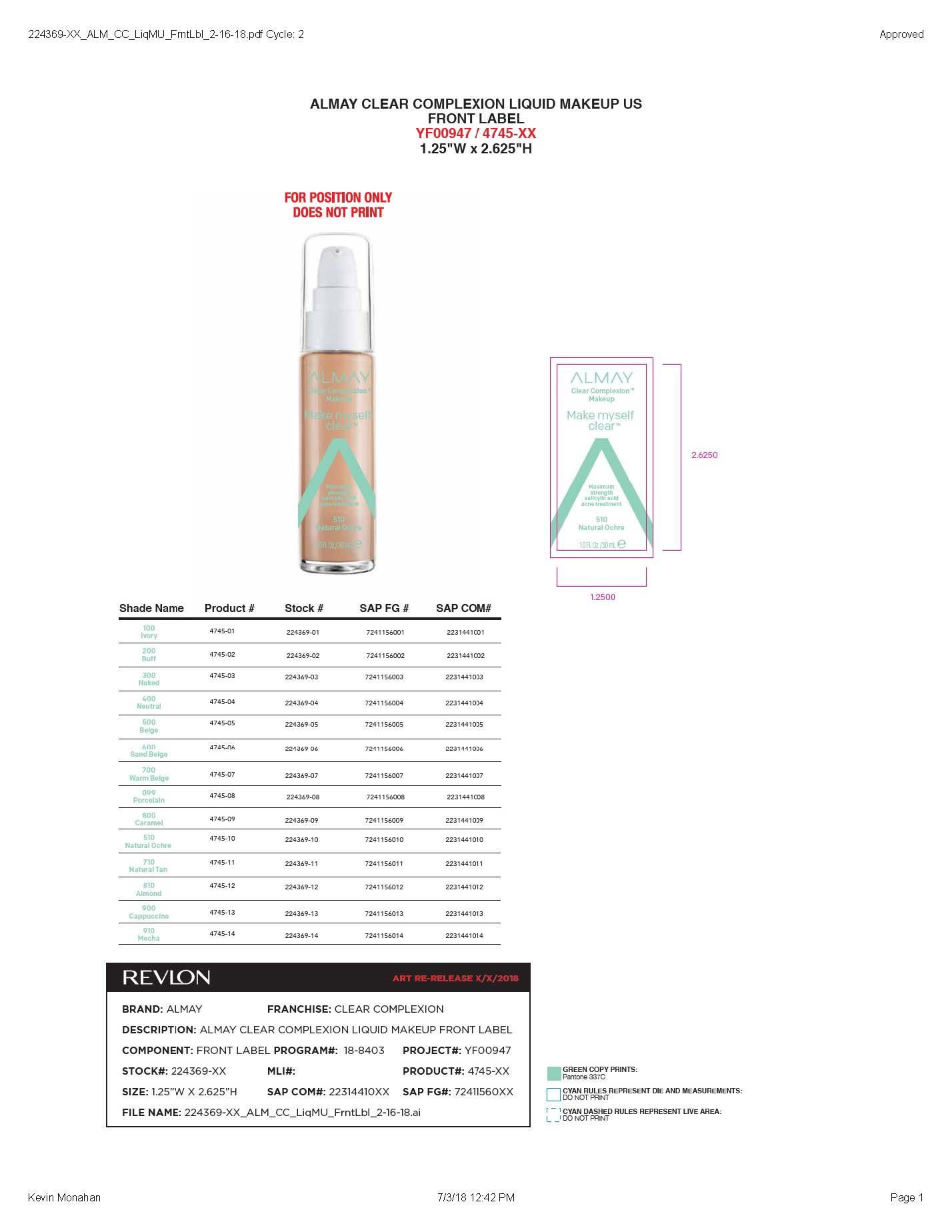
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALMAY CLEAR COMPLEXION LIQUID MAKEUP
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0311-0720 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mg in 2 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 6 (UNII: XHK3U310BA) HYDROLYZED SOY PROTEIN (ENZYMATIC; 2000 MW) (UNII: 1394NXB9L6) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) NYLON-12 (UNII: 446U8J075B) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BORON NITRIDE (UNII: 2U4T60A6YD) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) MALTODEXTRIN (UNII: 7CVR7L4A2D) ETHYLHEXYL PALMITATE (UNII: 2865993309) PANTHENOL (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) METHYLPARABEN (UNII: A2I8C7HI9T) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SODIUM CHLORIDE (UNII: 451W47IQ8X) MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROGEN DIMETHICONE (20 CST) (UNII: 12Z59IF64N) PANTHENYL ETHYL ETHER (UNII: F4WMF8NX3B) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) ETHYLPARABEN (UNII: 14255EXE39) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0311-0720-01 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/23/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 04/23/2018 Labeler - Almay (064988652) Establishment Name Address ID/FEI Business Operations REVLON, INC. 809725570 manufacture(0311-0720)

 up
up