Label: GILTUSS TR- guaifenesin,dextromethorphan hbr,phenylephrine hcl tablet
- NDC Code(s): 58552-335-01, 58552-335-02
- Packager: Gil Pharmaceutical Corp
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 24, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
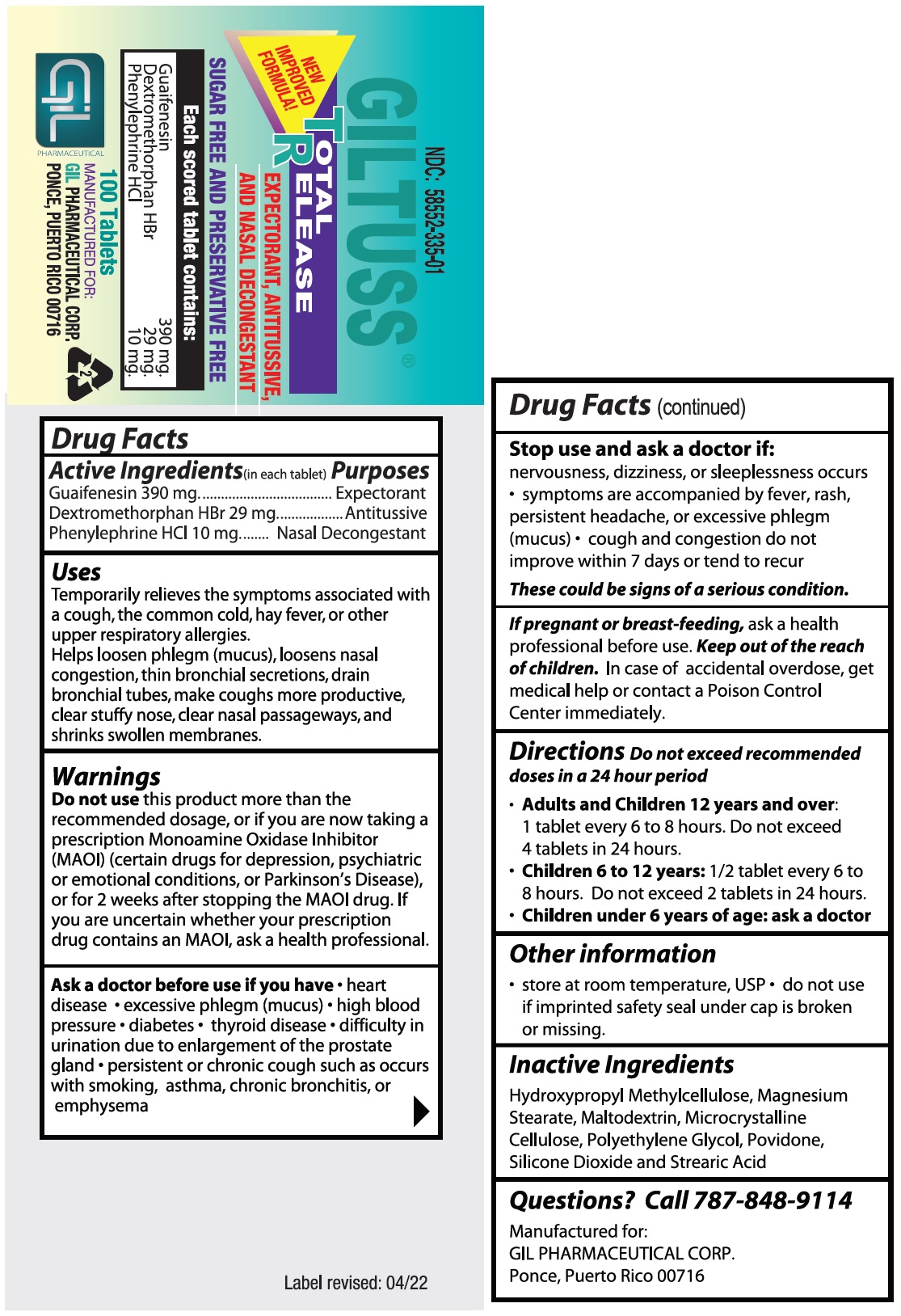
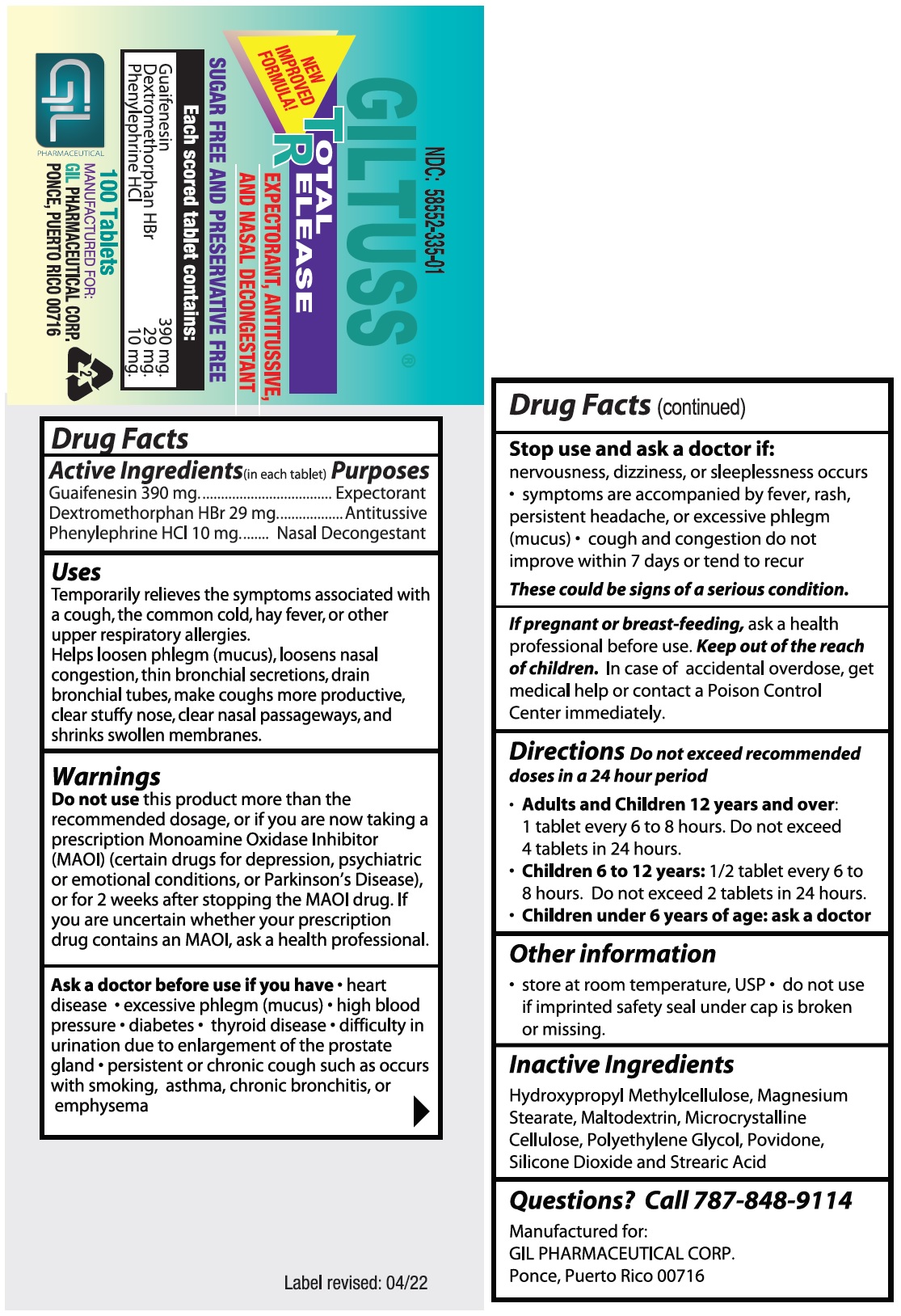
- Drug Facts
- Purposes
-
Uses
Temporarily relieves the symptoms associated with a cough, the common cold, hay fever, or other upper respiratory allergies.
Helps loosen phlegm (mucus), loosens nasal congestion, thin bronchial secretions, drain bronchial tubes, make coughs more productive, clear stuffy nose, clear nasal passageways, and shrinks swollen membranes.
-
Warnings
Do not usethis product more than the recommended dosage, or if you are now taking a prescription Monoamine Oxidase Inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s Disease), or for 2 weeks after stopping the MAOI drug.
If you are uncertain whether your prescription drug contains an MAOI, ask a health professional.
- Ask a doctor before use if you have
- Stop use and ask a doctor if:
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive Ingredients
- Questions? Call 787-848-9114
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GILTUSS TR
guaifenesin,dextromethorphan hbr,phenylephrine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58552-335 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 390 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 29 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE 10 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape OVAL Size 8mm Flavor Imprint Code 303;Gil Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58552-335-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/07/2022 2 NDC:58552-335-02 2 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/07/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/07/2022 Labeler - Gil Pharmaceutical Corp (176826592) Registrant - Syntho Pharmaceuticals, Inc (113616187) Establishment Name Address ID/FEI Business Operations Syntho Pharmaceuticals, Inc. 088797407 manufacture(58552-335)