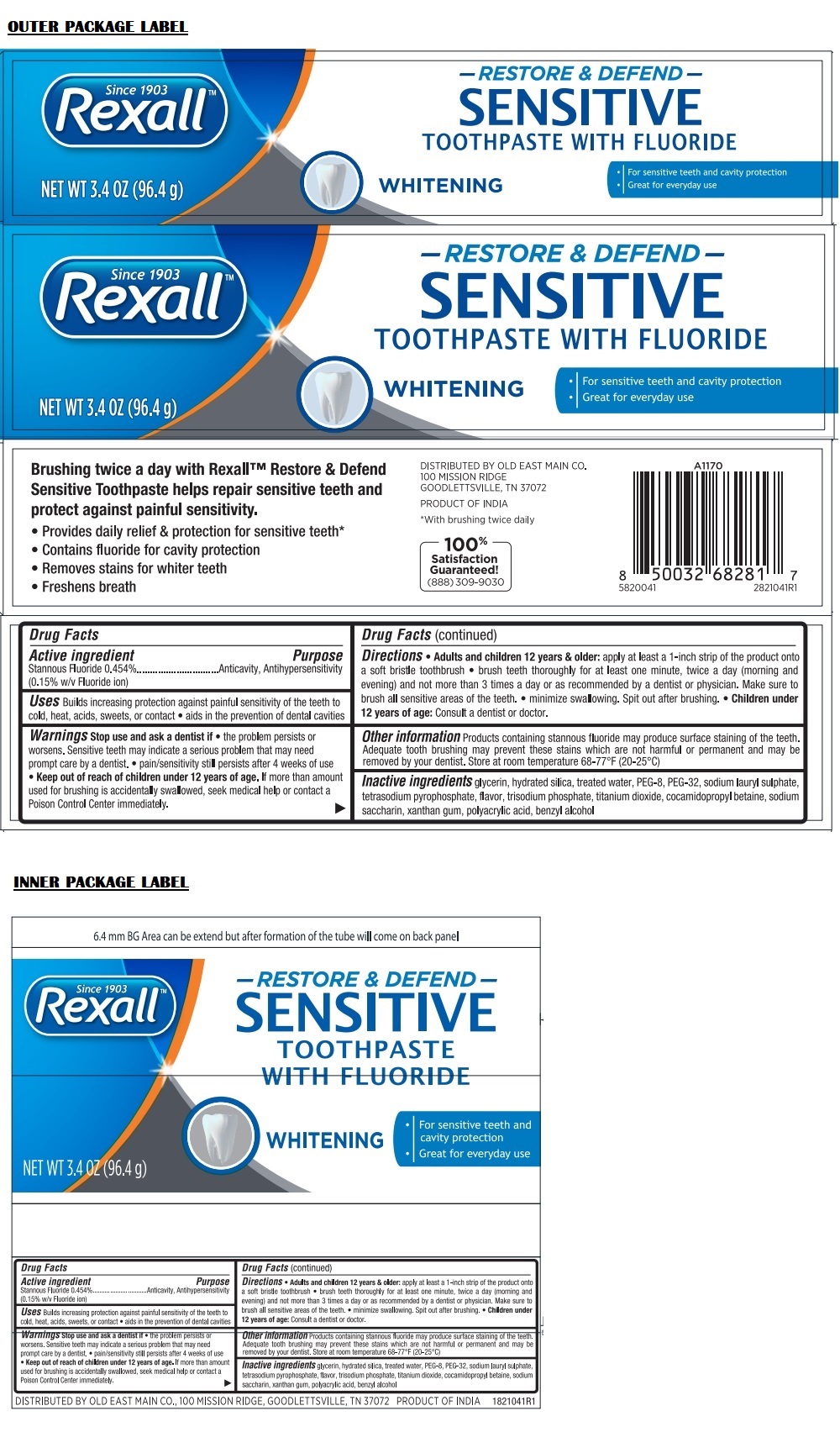
Label: REXALL RESTORE AND DEFEND SENSITIVE WHITENING- stannous fluoride paste, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 55910-820-01 - Packager: Old East Main CO.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 23, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
• Adult and children 12 years & older: apply at least a 1-inch strip of the product onto a soft bristle toothbrush • brush teeth thoroughly for at least one minute, twice a day (morning and evening) and not more than 3 times a day or as recommended by a dentist or physician. Make sure to brush all sensitive areas of the teeth. • minimize swallowing. Spit out after brushing. • Children under 12 years of age: Consult a dentist or doctor.
- Other information
- Inactive ingredients
-
SPL UNCLASSIFIED SECTION
Since 1903
TOOTHPASTE WITH FLUORIDE
• For sensitive teeth and cavity protection
• Great for everyday useBrushing twice a day with Rexall™ Restore & Defend Sensitive Toothpaste helps repair sensitive teeth and protect against painful sensitivity.
• Provides daily relief & protection for sensitive teeth*
• Contains fluoride for cavity protection
• Removes stains for whiter teeth
• Freshens breath100% Satisfaction Guaranteed!
(888) 309-9030*With brushing twice daily
DISTRIBUTED BY OLD EAST MAIN CO.
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072PRODUCT OF INDIA
- Packaging
-
INGREDIENTS AND APPEARANCE
REXALL RESTORE AND DEFEND SENSITIVE WHITENING
stannous fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-820 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 4.54 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WATER (UNII: 059QF0KO0R) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 1600 (UNII: 1212Z7S33A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) SODIUM PHOSPHATE, TRIBASIC, DODECAHYDRATE (UNII: B70850QPHR) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SACCHARIN SODIUM (UNII: SB8ZUX40TY) XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-820-01 1 in 1 BOX 03/21/2022 1 96.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 03/21/2022 Labeler - Old East Main CO. (068331990) Registrant - Ashtel Studios Inc. (148689180)