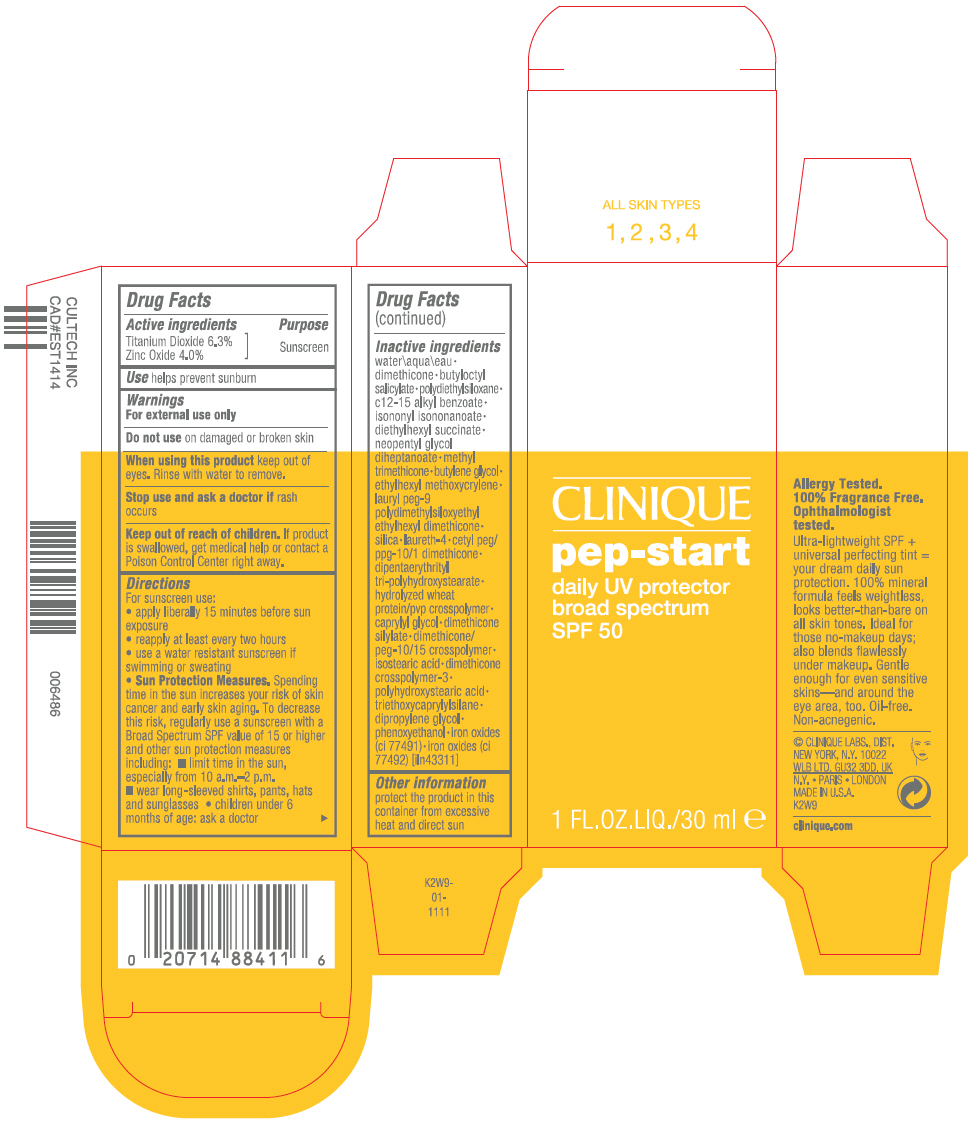
Label: PEP-STAR DAILY UV PROTECTOR BROAD SPECTRUM SPF 50- titanium dioxide and zinc oxide lotion
- NDC Code(s): 49527-073-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 13, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau • dimethicone • butyloctyl salicylate • polydiethylsiloxane • c12-15 alkyl benzoate • isononyl isononanoate • diethylhexyl succinate • neopentyl glycol diheptanoate • methyl trimethicone • butylene glycol • ethylhexyl methoxycrylene • lauryl peg-9 polydimethylsiloxyethyl ethylhexyl dimethicone • silica • laureth-4 • cetyl peg/ppg-10/1 dimethicone • dipentaerythrityl tri-polyhydroxystearate • hydrolyzed wheat protein/pvp crosspolymer • caprylyl glycol • dimethicone silylate • dimethicone/peg-10/15 crosspolymer • isostearic acid • dimethicone crosspolymer-3 • polyhydroxystearic acid • triethoxycaprylylsilane • dipropylene glycol • phenoxyethanol • iron oxides (ci 77491) • iron oxides (ci 77492) [iln43311]
- Other information
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
PEP-STAR DAILY UV PROTECTOR BROAD SPECTRUM SPF 50
titanium dioxide and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-073 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 63 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) DIETHYLHEXYL SUCCINATE (UNII: 69W9UMG3P8) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LAURETH-4 (UNII: 6HQ855798J) DIPENTAERYTHRITYL TRI-POLYHYDROXYSTEARATE (UNII: D21K655H52) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISOSTEARIC ACID (UNII: X33R8U0062) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPROPYLENE GLYCOL (UNII: E107L85C40) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-073-01 1 in 1 CARTON 01/01/2017 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2017 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(49527-073) , pack(49527-073) , label(49527-073)