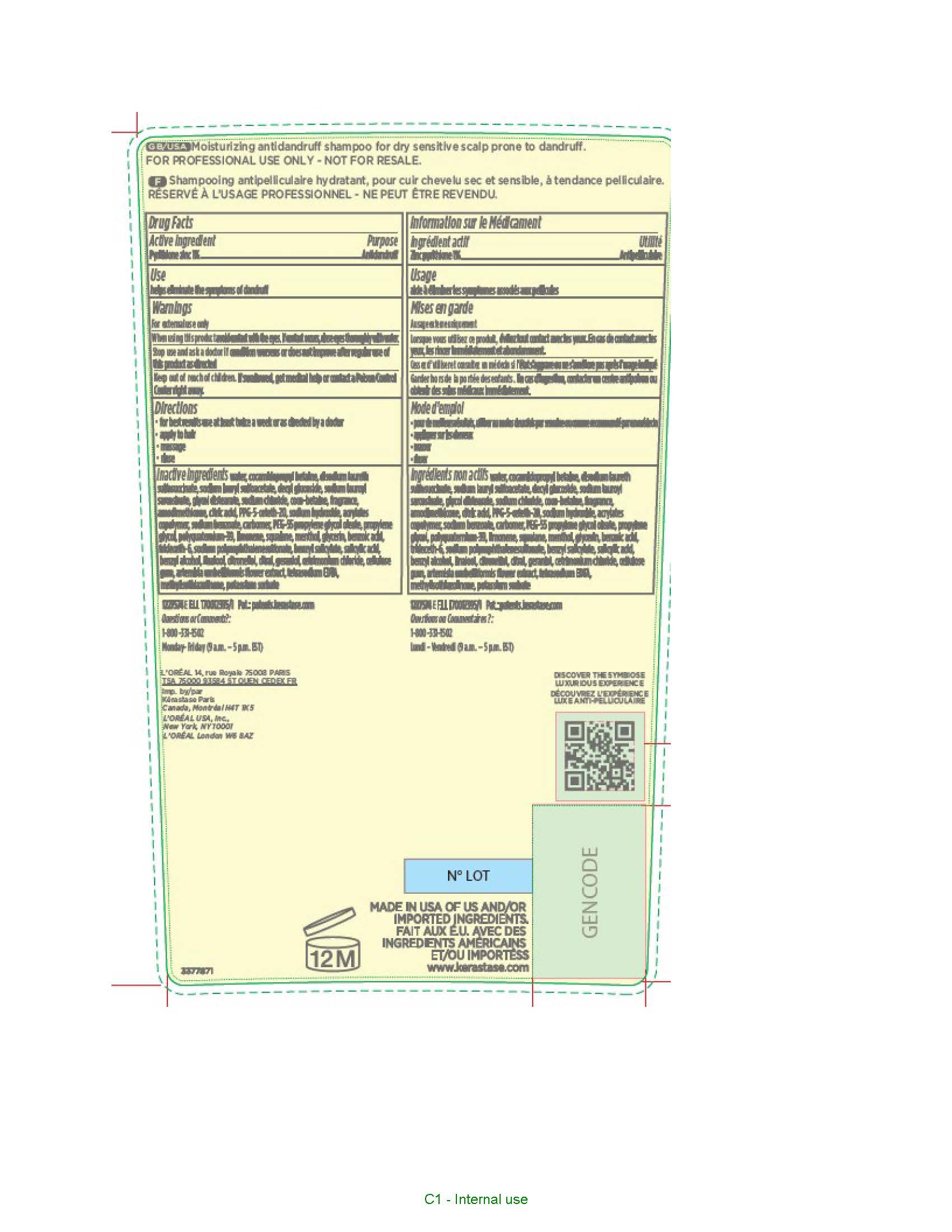
Label: KERASTASE PARIS SYMBIOSE ANTIDANDRUFF- pyrithione zinc shampoo
- NDC Code(s): 49967-871-01, 49967-871-02, 49967-871-03
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 24, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
-
Inactive ingredients
water, cocamidopropyl betaine, disodium laureth sulfosuccinate, sodium lauryl sulfoacetate, decyl glucoside, sodium lauroyl sarcosinate, glycol distearate, sodium chloride, coco-betaine, fragrance, amodimethicone, citric acid, PPG-5-ceteth-20, sodium hydroxide, acrylates copolymer, sodium benzoate, carbomer, PEG-55 propylene glycol oleate, propylene glycol, polyquaternium-39, limonene, squalane, menthol, glycerin, benzoic acid, trideceth-6, sodium polynaphthalenesulfonate, benzyl salicylate, salicylic acid, benzyl alcohol, linalool, citronellol, citral, geraniol, cetrimonium chloride, cellulose gum, artemisia umbelliformis flower extract, tetrasodium EDTA, methylisothiazolinone, potassium sorbate
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KERASTASE PARIS SYMBIOSE ANTIDANDRUFF
pyrithione zinc shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-871 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) SODIUM LAURYL SULFOACETATE (UNII: D0Y70F2B9J) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) GLYCOL DISTEARATE (UNII: 13W7MDN21W) SODIUM CHLORIDE (UNII: 451W47IQ8X) COCO-BETAINE (UNII: 03DH2IZ3FY) AMODIMETHICONE (1300 CST) (UNII: 3V7U636DWN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PPG-5-CETETH-20 (UNII: 4AAN25P8P4) SODIUM HYDROXIDE (UNII: 55X04QC32I) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) SODIUM BENZOATE (UNII: OJ245FE5EU) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PEG-55 PROPYLENE GLYCOL OLEATE (UNII: 7RDE7PJS40) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYQUATERNIUM-39 (35/35/30 ACRYLIC ACID/ACRYLAMIDE/DADMAC; 1500000 MW) (UNII: EOD3UE785A) LIMONENE, (+)- (UNII: GFD7C86Q1W) SQUALANE (UNII: GW89575KF9) MENTHOL (UNII: L7T10EIP3A) GLYCERIN (UNII: PDC6A3C0OX) BENZOIC ACID (UNII: 8SKN0B0MIM) TRIDECETH-6 (UNII: 3T5PCR2H0C) BENZYL SALICYLATE (UNII: WAO5MNK9TU) SALICYLIC ACID (UNII: O414PZ4LPZ) BENZYL ALCOHOL (UNII: LKG8494WBH) LINALOOL, (+/-)- (UNII: D81QY6I88E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) CITRAL (UNII: T7EU0O9VPP) GERANIOL (UNII: L837108USY) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) ARTEMISIA UMBELLIFORMIS FLOWER (UNII: 91OLL9AJ7D) EDETATE SODIUM (UNII: MP1J8420LU) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-871-01 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/12/2022 01/31/2025 2 NDC:49967-871-02 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/12/2022 04/30/2025 3 NDC:49967-871-03 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/12/2022 01/31/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 12/12/2022 04/30/2025 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'OREAL USA, INC 960317444 manufacture(49967-871) , pack(49967-871)