Label: CLOSYS- sodium fluoride paste, dentifrice
- NDC Code(s): 58578-0749-1, 58578-0749-2, 58578-0749-3
- Packager: Rowpar Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 22, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT:
- PURPOSE:
- USE:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS:
adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
children under 6 years of age:
- instruct in good brushing and rinsing habits (to minimize swallowing).
- supervise children as necessary until capable of using without supervision.
- do not swallow
children under 2 years of age:
consult a dentist or doctor
- INACTIVE INGREDIENTS:
- SPL UNCLASSIFIED SECTION
- Questions or comments?
-
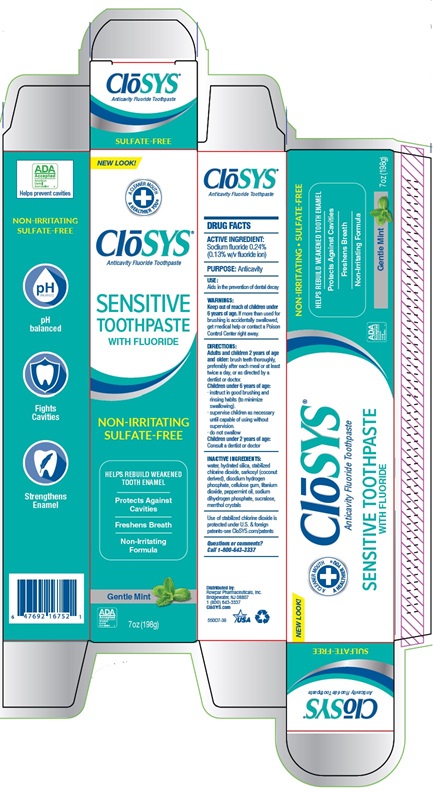
Principal Display Panel
Closys®
Anticavity Fluoride Toothpaste
SULFATE-FREE
NEW LOOK!
A CLEANER MOUTH
A HEALTHIER YOU®
CLoSYS ®
Anticavity Fluoride Toothpaste
SENSITIVE
TOOTHPASTE
WITH FLUORIDE
NON-IRRITATING
SULFATE-FREE
HELPS REBUILD WEAKEND
TOOTH ENAMEL
Protects Against
Cavities
Freshens Breath
Non-Irritating
Formula
Gentle Mint
7oz (196g)
NON-IRRITATING
SULFATE-FREE
pH balanced
Fights
Cavities
Strengthens
Enamel
Closys®
Anticavity Fluoride Toothpaste
SENSITIVE TOOTHPASTE
WITH FLUORIDE
NON-IRRITATING SULFATE-FREE
HELPS REBUILD WEAKEND TOOTH ENAMEL
Protects Against Cavities
Freshens Breath
Non-Irritating Formula
Gentle Mint 7oz (196g)
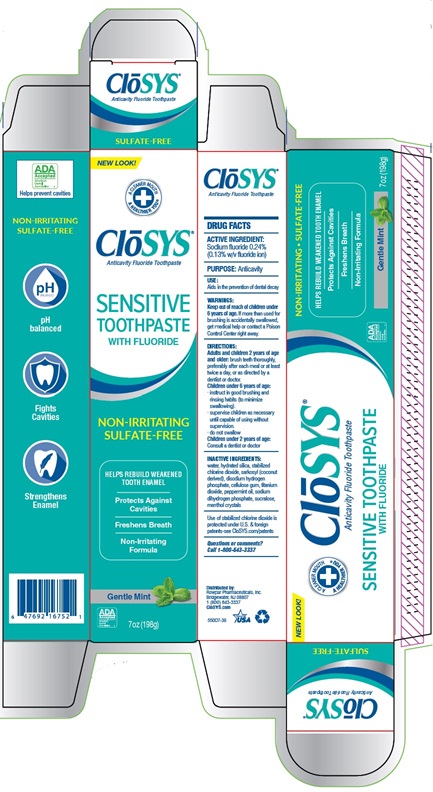
Closys®
Anticavity Fluoride Toothpaste
SULFATE-FREE
NEW LOOK!
A CLEANER MOUTH
A HEALTHIER YOU®
CLoSYS ®
Anticavity Fluoride Toothpaste
SENSITIVE
TOOTHPASTE
WITH FLUORIDE
NON-IRRITATING
SULFATE-FREE
HELPS REBUILD WEAKEND
TOOTH ENAMEL
Protects Against
Cavities
Freshens Breath
Non-Irritating
Formula
Gentle Mint
3.4oz (96g)
NON-IRRITATING
SULFATE-FREE
pH balanced
Fights
Cavities
Strengthens
Enamel
Closys®
Anticavity Fluoride Toothpaste
SENSITIVE TOOTHPASTE
WITH FLUORIDE
NON-IRRITATING SULFATE-FREE
HELPS REBUILD WEAKEND TOOTH ENAMEL
Protects Against Cavities
Freshens Breath
Non-Irritating Formula
Gentle Mint 3.4oz (96g)

-
INGREDIENTS AND APPEARANCE
CLOSYS
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58578-0749 Route of Administration ORAL, DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.1 mg in 1 g Inactive Ingredients Ingredient Name Strength SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYDRATED SILICA (UNII: Y6O7T4G8P9) CHLORINE DIOXIDE (UNII: 8061YMS4RM) PEPPERMINT OIL (UNII: AV092KU4JH) MENTHOL (UNII: L7T10EIP3A) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) Product Characteristics Color white Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58578-0749-1 1 in 1 BOX 10/20/2017 1 96 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:58578-0749-2 1 in 1 BOX 10/20/2017 2 198 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:58578-0749-3 1 in 1 BOX 10/20/2017 06/30/2025 3 21 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 10/20/2017 Labeler - Rowpar Pharmaceuticals, Inc. (783704661) Establishment Name Address ID/FEI Business Operations Transom Symphony OPCO, LLC 117501991 manufacture(58578-0749)