Label: DERMELEVE- pramoxine hydrochloride cream
- NDC Code(s): 81507-005-01, 81507-005-02
- Packager: Advanced Derm Solutions LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 16, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
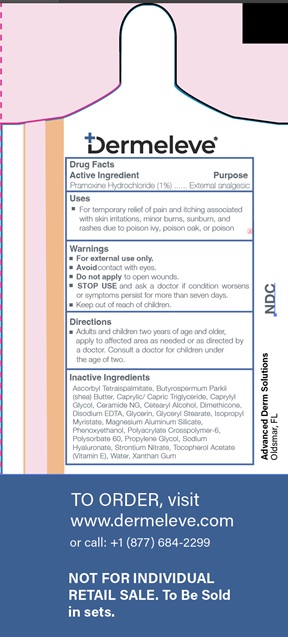
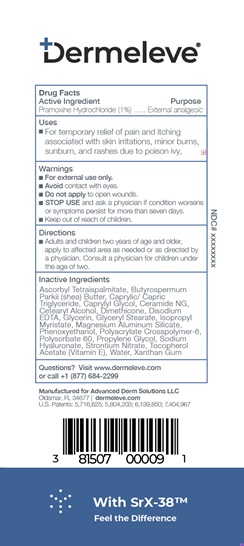
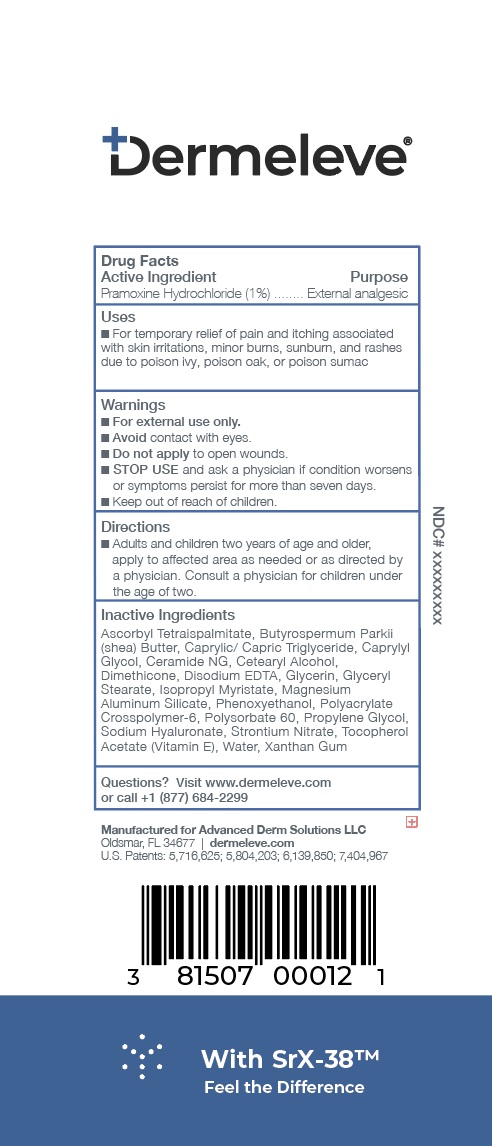
- Active Ingredient
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
Inactive ingredients
Ascorbyl Tetraispalmitate, Butyrospermum Parkii (shea) Butter, Caprylic/Capric Triglyceride, Caprylyl Glycol, Ceramide NG, Cetearyl Alcohol
Dimethicone, Disodium EDTA, Glycerin, Glyceryl Stearate, Isopropyl Myristate, Magnesium Aluminum Silicate, Phenoxyethanol, Polyacrylate Crosspolymer-6, Polysorbate 60, Propylene Glycol, Sodium Hyaluronate, Strontium Nitrate, Tocopherol Acetate (Vitamin E), Water, Xanthan Gum - Product label
-
INGREDIENTS AND APPEARANCE
DERMELEVE
pramoxine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81507-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 1 g in 100 g Inactive Ingredients Ingredient Name Strength ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHICONE 100 (UNII: RO266O364U) POLYACRYLIC ACID (450000 MW) (UNII: KD3S7H73D3) HYALURONIC ACID (UNII: S270N0TRQY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLAWAX POLYSORBATE (UNII: Q504PL8E0V) GLYCERIN (UNII: PDC6A3C0OX) CAPRYLYL GLYCOL (UNII: 00YIU5438U) XANTHAN GUM (UNII: TTV12P4NEE) CERAMIDE NG (UNII: C04977SRJ5) WATER O-18 (UNII: 7QV8F8BYNJ) MAGNESIUM ALUMINUM SILICATE TYPE IA (UNII: SUS08ZOA9S) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) EDETATE DISODIUM (UNII: 7FLD91C86K) TOCOPHEROL (UNII: R0ZB2556P8) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) SHEA BUTTER (UNII: K49155WL9Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) STRONTIUM NITRATE (UNII: BDG873AQZL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81507-005-01 1 in 1 BOX 11/01/2024 1 60 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:81507-005-02 1 in 1 BOX 11/01/2024 2 120 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 11/01/2024 Labeler - Advanced Derm Solutions LLC (117840544)