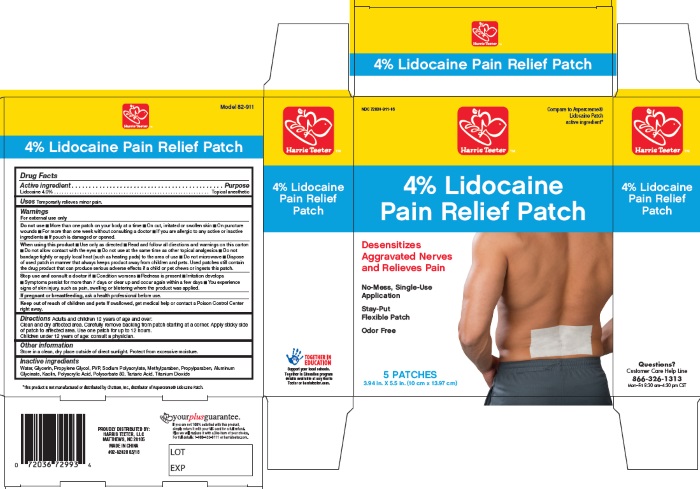
Label: PAIN RELIEF PATCHES- lidocaine patch
- NDC Code(s): 72036-911-05
- Packager: Harris Teeter
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Dosage and Administration
- Warnings
- Idications and Usage
-
When using this product
■ use only as directed
■ read and follow all directions and warnings on this carton
■ do not allow contact with the eyes
■ do not use at the same time as other topical analgesics
■ do not bandage tightly or apply local heat (such as heating pads) to the area of use
■ do not microwave
■ dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
- Stop use and consult a doctor
- PREGNANCY OR BREAST FEEDING
- Do not Use
- Keep out of reach of children and pets.
- Other Safety Information
- Inactive Ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF PATCHES
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72036-911 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength ALUMINUM (UNII: CPD4NFA903) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) POLYACRYLIC ACID (300000 MW) (UNII: A8371R0U5J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72036-911-05 5 in 1 CARTON 05/01/2018 1 9 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/01/2018 Labeler - Harris Teeter (047279351) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co.,Ltd. 529128763 manufacture(72036-911)