Label: FOUNDATION PRIMER PLUS SPF 15 MERLE NORMAN- octinoxate, octisalate cream
- NDC Code(s): 57627-108-01, 57627-108-02, 57627-108-03, 57627-108-04
- Packager: Merle Norman Cosmetics
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 20, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Skin Cancer/Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin canceror early skin aging.
For external use only.
Do not use on damaged or Broken skin
When using this product
keep out of eyes. Rinse with water to remove.
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients
Cyclopentasiloxane, Dimethicone Crosspolymer, Trisiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Cyclomethicone, Palmitoyl Oligopeptide, Ascorbyl Palmitate, Retinyl Palmitate, Tocopheryl Acetate, Ceramide 2 , Camellia Oleifera Leaf Extract, PEG-10 Rapeseed Sterol, Phospholipids, C12-15 Alkyl Benzoate, Cyclotetrasiloxane, Water (Aqua), Isoceteth-10, Tribehenin, Butylene Glycol, Silica, Phenoxyethanol.
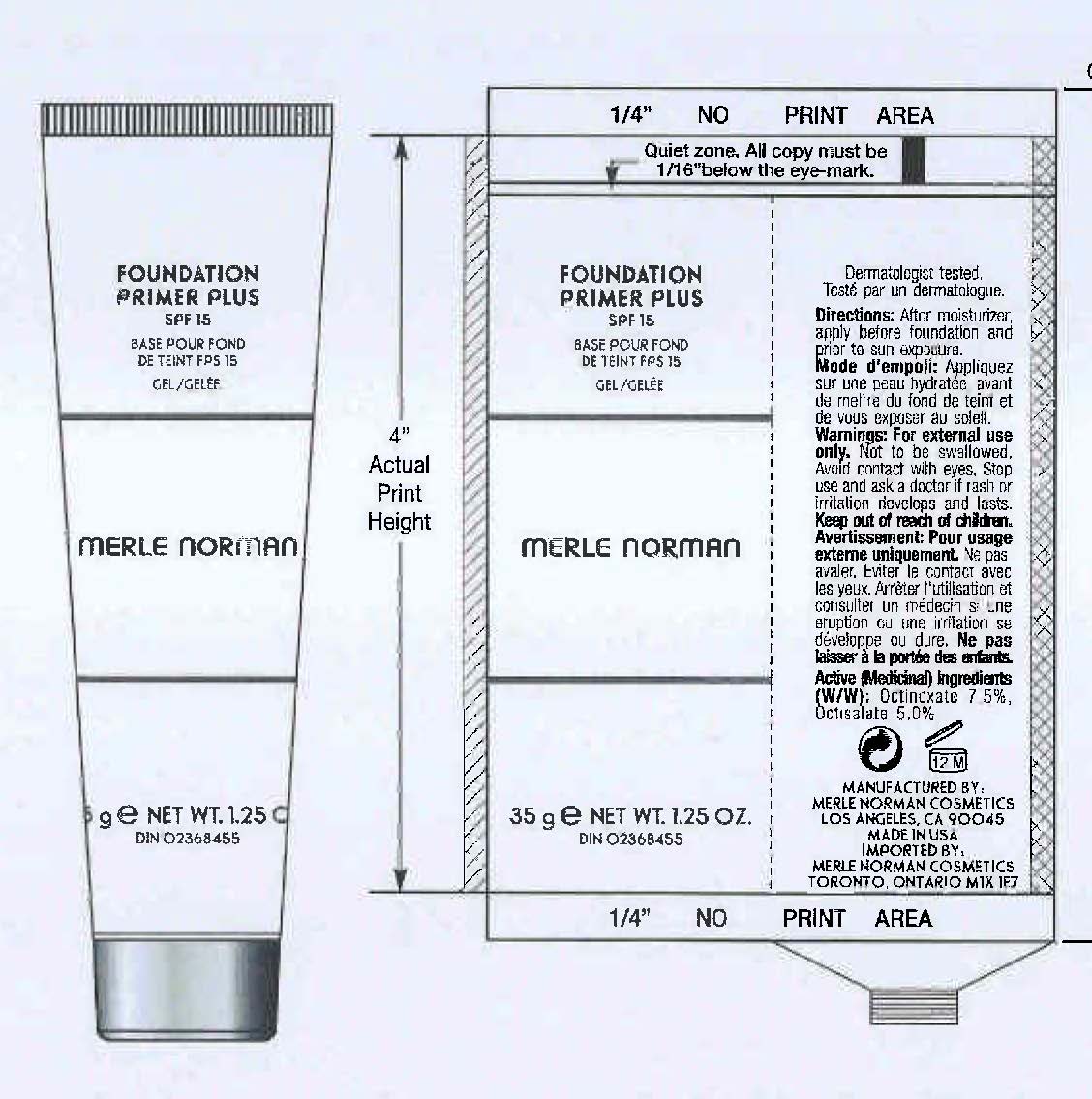
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOUNDATION PRIMER PLUS SPF 15 MERLE NORMAN
octinoxate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57627-108 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.63 g in 35 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 1.75 g in 35 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) TRISILOXANE (UNII: 9G1ZW13R0G) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) ASCORBYL PALMITATE (UNII: QN83US2B0N) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) CERAMIDE 2 (UNII: C04977SRJ5) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) PEG-10 RAPESEED STEROL (UNII: 258O76T85M) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) TRIBEHENIN (UNII: 8OC9U7TQZ0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57627-108-02 1 in 1 BOX 04/22/2011 1 NDC:57627-108-01 35 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:57627-108-04 1 in 1 BOX 04/22/2011 2 NDC:57627-108-03 5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/22/2011 Labeler - Merle Norman Cosmetics (008479388) Registrant - Merle Norman Cosmetics (008479388) Establishment Name Address ID/FEI Business Operations Merle Norman Cosmetics 008479388 manufacture(57627-108)