Label: COLGATE TOTAL GUM PROTECT FRESH MINT- stannous fluoride paste, dentifrice
- NDC Code(s): 35000-278-63
- Packager: Colgate-Palmolive Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 3, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purposes
- Uses
-
Warnings
When using this product for sensitivity, do not use longer than 4 weeks unless recommended by a dentist.
Stop use and ask a dentist if the sensitivity problem persists or worsens. Sensitive teeth may indicate a serious problem that may need prompt care by a dentist.
-
Directions
- adults and children 12 years of age and older: apply at least a 1-inch strip of the product onto a soft bristled toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist. Make sure to brush all sensitive areas of the teeth.
- do not swallow.
- children under 12 years of age: consult a dentist.
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
Colgate®
Anticavity, Antigingivitis and Antisensitivity ToothpasteTotal
ACTIVE PREVENTIONGum
ProtectEliminates 100%
More Bacteria*
Helps Prevent Early
Gum Disease
SLS FREENET WT 3.0 OZ (85 g)
FRESH MINT
PASTECLINICALLY
PROVENANTIBACTERIAL
24
HOUR1✓Redness ✓Gum Bleeding ✓Gingivitis
✓Plaque ✓Cavities ✓Sensitivity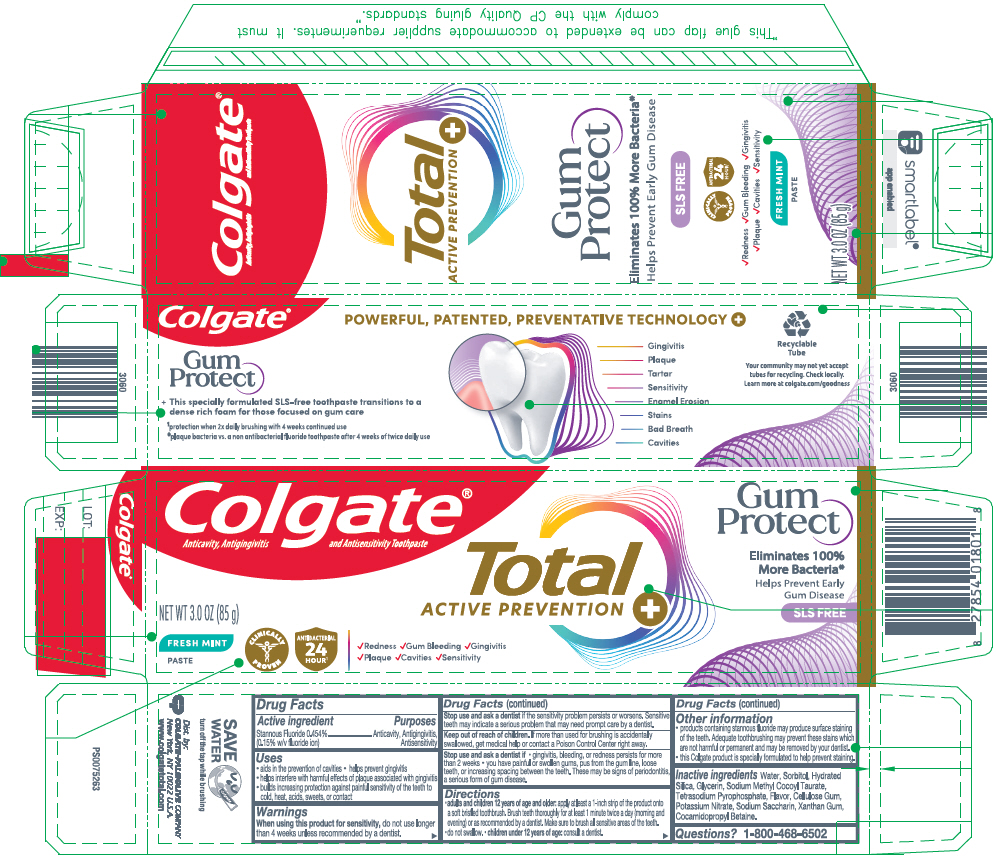
-
INGREDIENTS AND APPEARANCE
COLGATE TOTAL GUM PROTECT FRESH MINT
stannous fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35000-278 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.1 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) HYDRATED SILICA (UNII: Y6O7T4G8P9) GLYCERIN (UNII: PDC6A3C0OX) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) POTASSIUM NITRATE (UNII: RU45X2JN0Z) SACCHARIN SODIUM (UNII: SB8ZUX40TY) XANTHAN GUM (UNII: TTV12P4NEE) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color WHITE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35000-278-63 1 in 1 CARTON 10/18/2024 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M021 10/18/2024 Labeler - Colgate-Palmolive Company (001344381)

