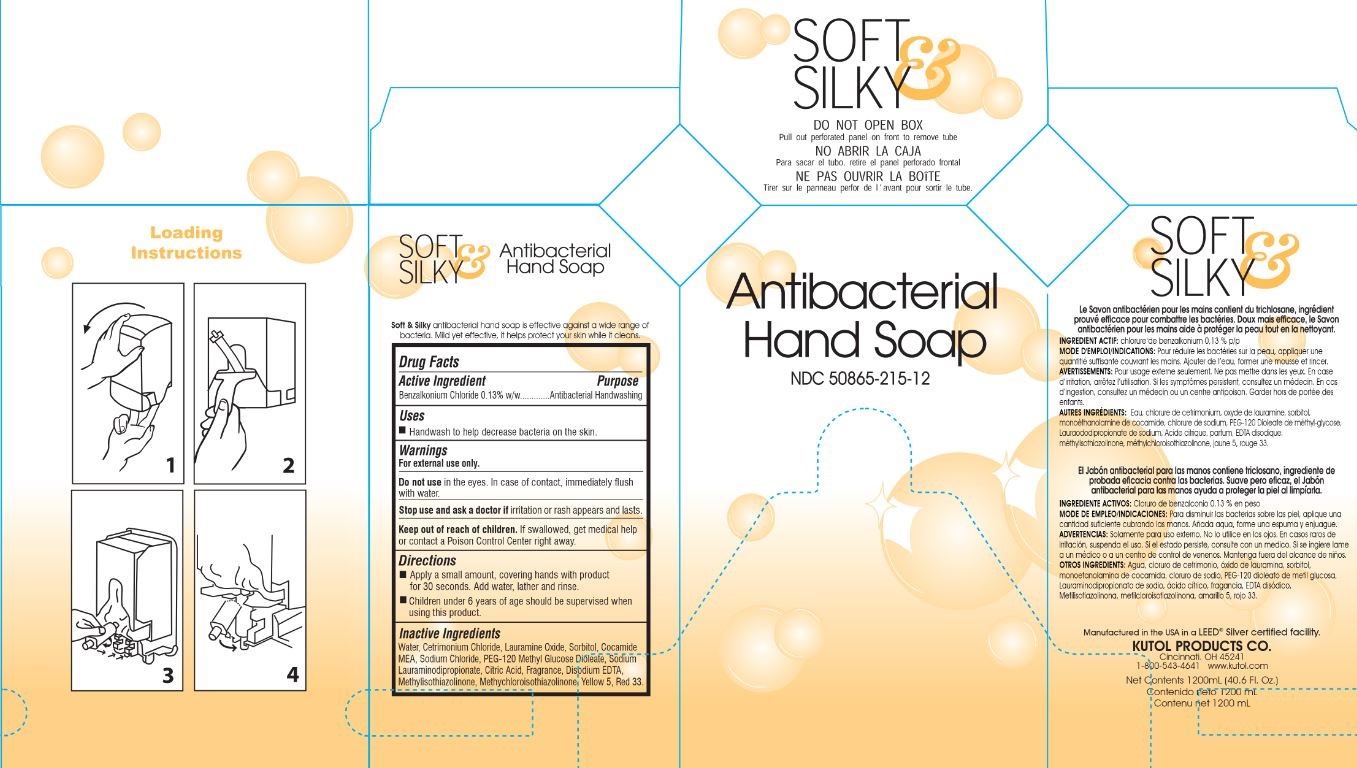
Label: ANTIBACTERIAL HAND CLEANSER- benzalkonium chloride soap
- NDC Code(s): 50865-215-09, 50865-215-12, 50865-215-19, 50865-215-65
- Packager: Kutol Products Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 28, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- WARNINGS
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL HAND CLEANSER
benzalkonium chloride soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50865-215 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.013 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) SORBITOL (UNII: 506T60A25R) COCO MONOETHANOLAMIDE (UNII: C80684146D) SODIUM LAURIMINODIPROPIONATE (UNII: 7G447D0DH9) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50865-215-09 3785 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/17/2017 2 NDC:50865-215-12 1200 mL in 1 BAG; Type 0: Not a Combination Product 08/17/2017 3 NDC:50865-215-19 237 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/17/2017 4 NDC:50865-215-65 800 mL in 1 BAG; Type 0: Not a Combination Product 08/17/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/17/2017 Labeler - Kutol Products Company (004236139) Registrant - Kutol Products Company (004236139) Establishment Name Address ID/FEI Business Operations Kutol Products Company 004236139 manufacture(50865-215)