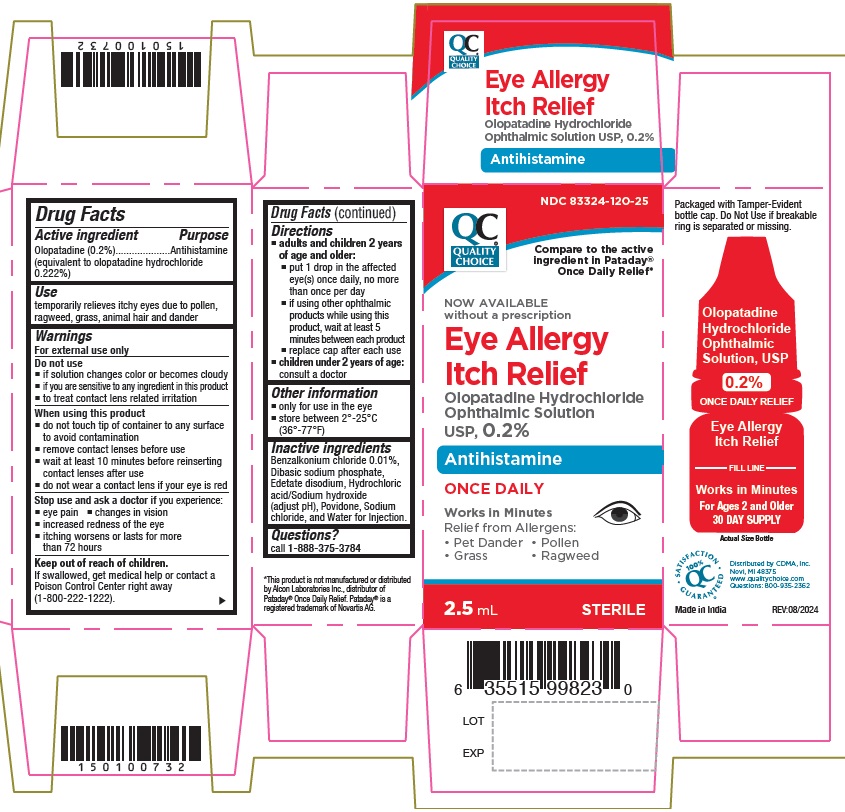
Label: OLOPATADINE HYDROCHLORIDE OPHTHALMIC SOLUTION- olopatadine hydrochloride ophthalmic solution
- NDC Code(s): 83324-120-25
- Packager: Chain Drug Marketing Association INC
- This is a repackaged label.
- Source NDC Code(s): 43598-764
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 3, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTOlopatadine (0.2%) (equivalent to olopatadine hydrochloride 0.222%)
-
PURPOSEAntihistamine
-
USEtemporarily relieves itchy eyes due to pollen, ragweed, grass, animal hair and dander
-
WARNINGSFor external use only
-
DO NOT USEif solution changes color or becomes cloudy if you are sensitive to any ingredient in this product - to treat contact lens related irritation
-
WHEN USING THIS PRODUCTdo not touch tip of container to any surface to avoid contamination - remove contact lenses before use - wait at least 10 minutes before reinserting contact lenses after use - do not wear a contact ...
-
STOP USE AND ASK DOCTOR IFyou experience: eye pain - changes in vision - increased redness of the eye - itching worsens or lasts for more than 72 hours
-
KEEP OUT OF REACH OF CHILDRENIf swallowed, get medical help or contact a Poison Control Center right away.
-
DIRECTIONSadults and children 2 years of age and older: put 1 drop in the affected eye(s) once daily, no more than once per day - if using other ophthalmic products while using this product, wait at least ...
-
OTHER INFORMATION• only for use in the eye • store between 2° to 25°C (36° to 77°F)
-
INACTIVE INGREDIENTSBenzalkonium chloride 0.01%, Dibasic sodium phosphate, Edetate disodium, Hydrochloric acid/Sodium hydroxide (adjust pH), Povidone, Sodium chloride, and Water for Injection.
-
QUESTIONS?Call 1-888-375-3784
-
PRINCIPAL DISPLAY PANEL(What is this?)NDC 83324-120-25 - Olopatadine Hydrochloride - Ophthalmic Solution, USP - 0.2% Bottle Label: Olopatadine Hydrochloride - Ophthalmic Solution, USP - 0.2% Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information