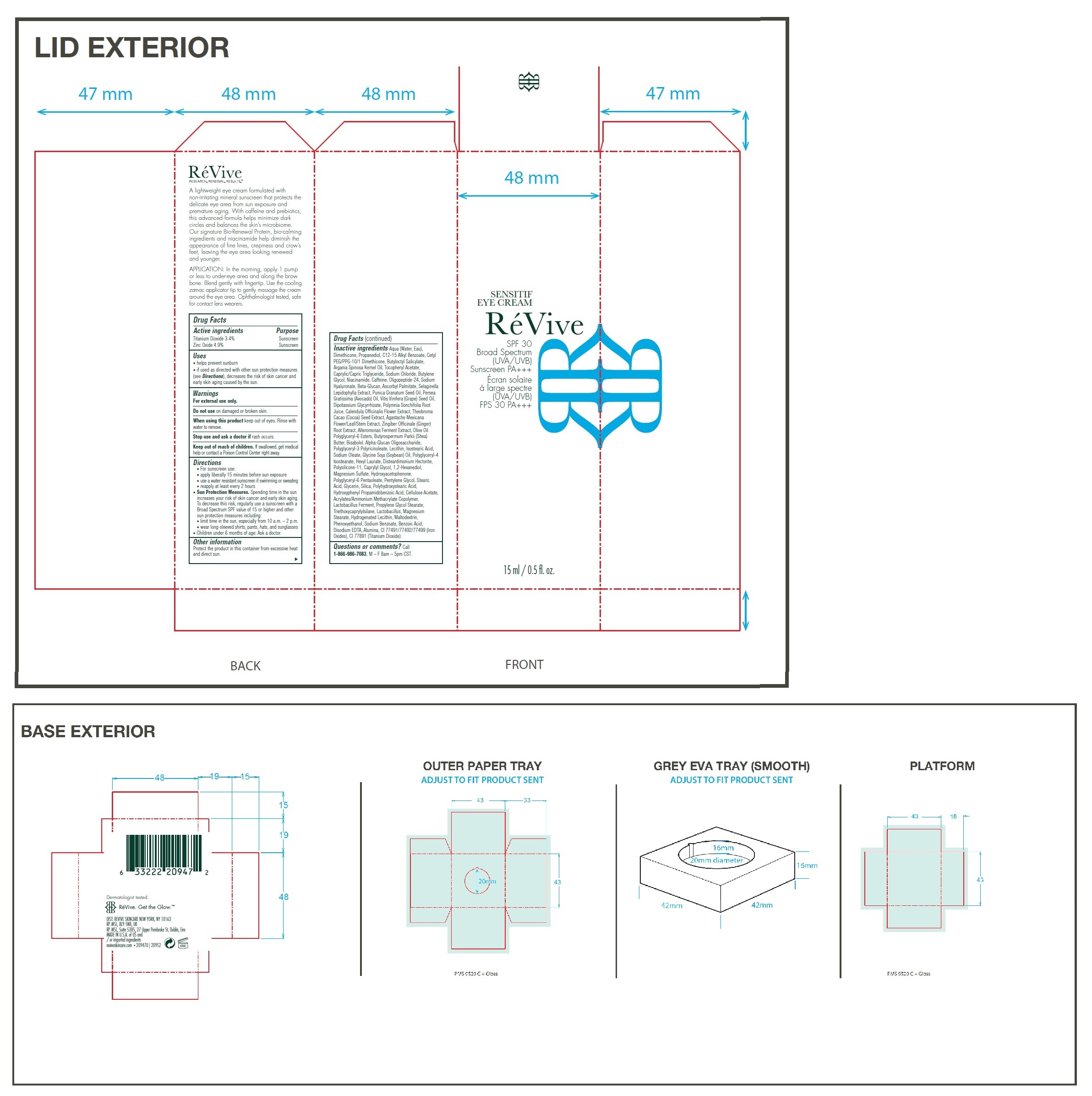
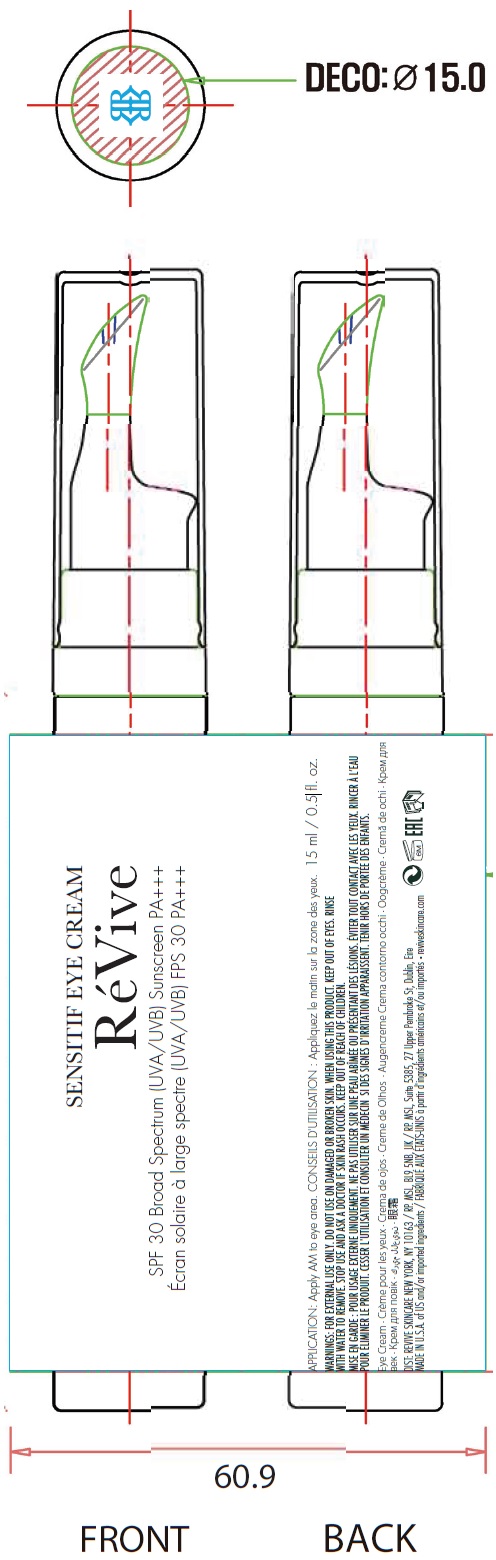
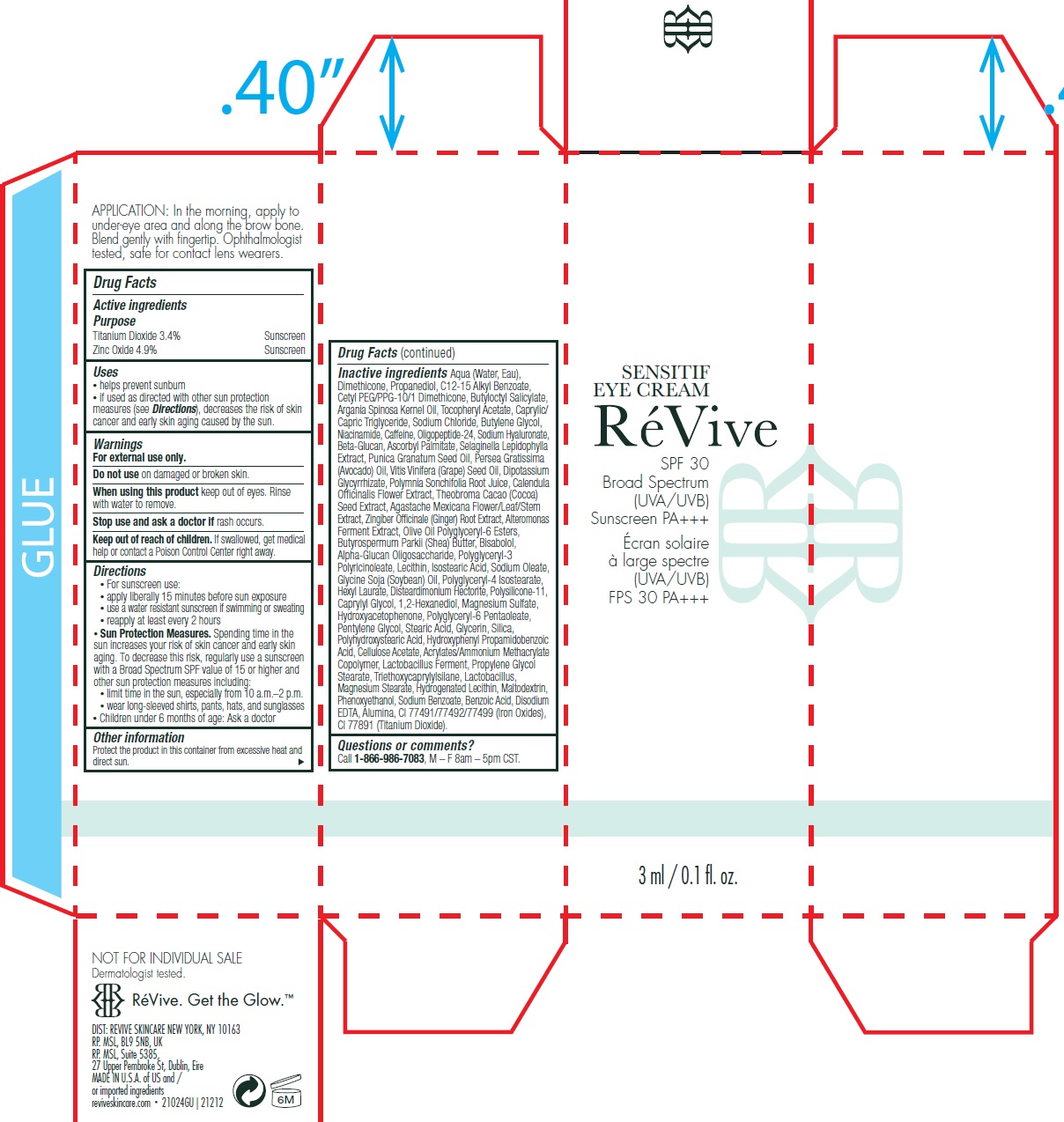
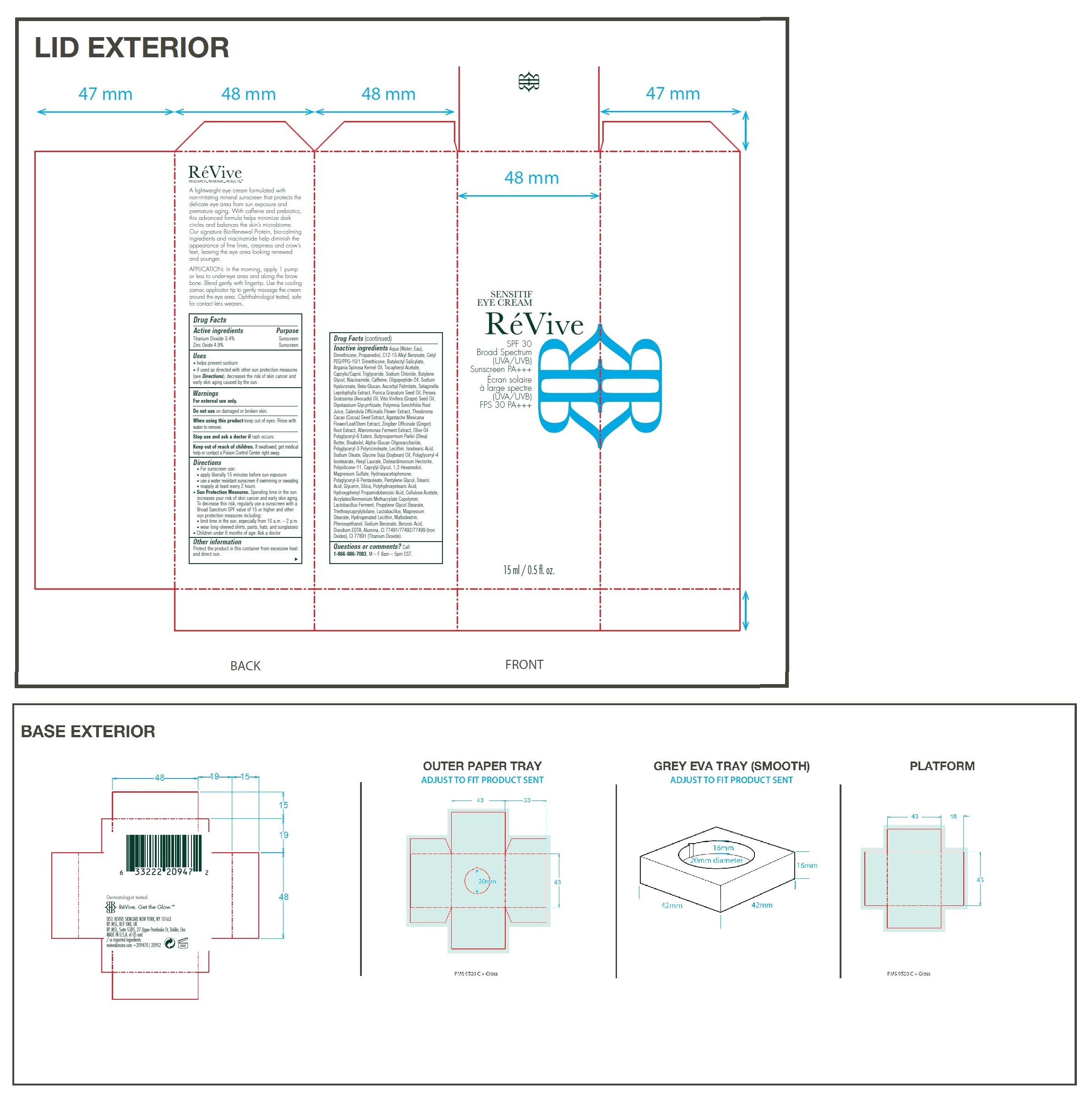
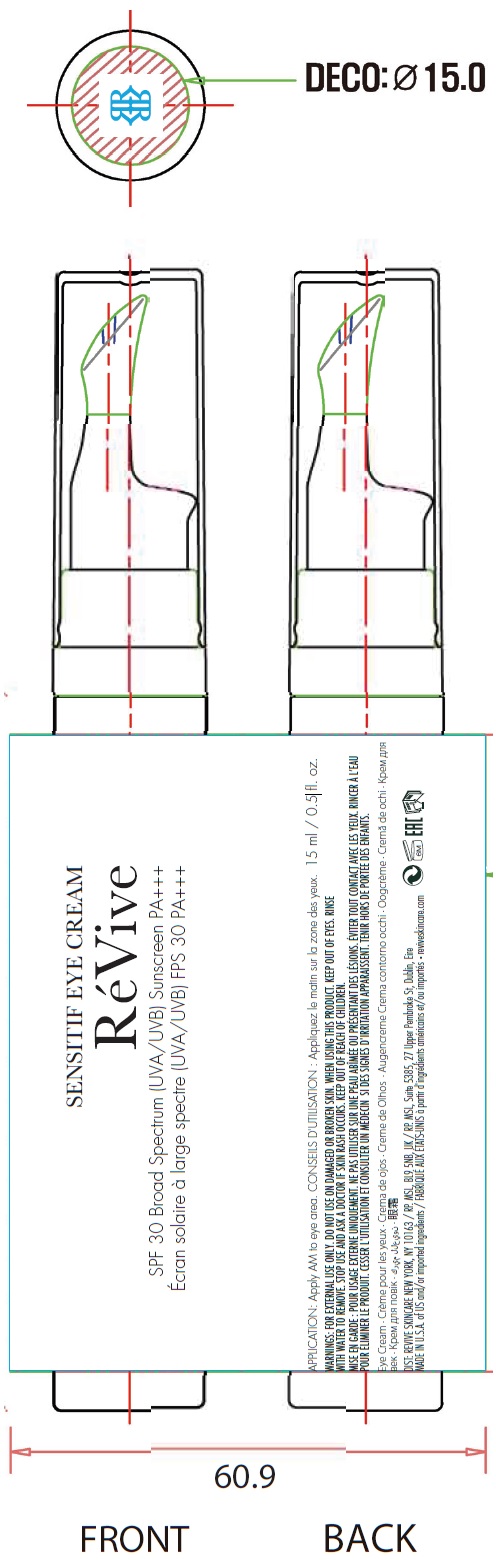
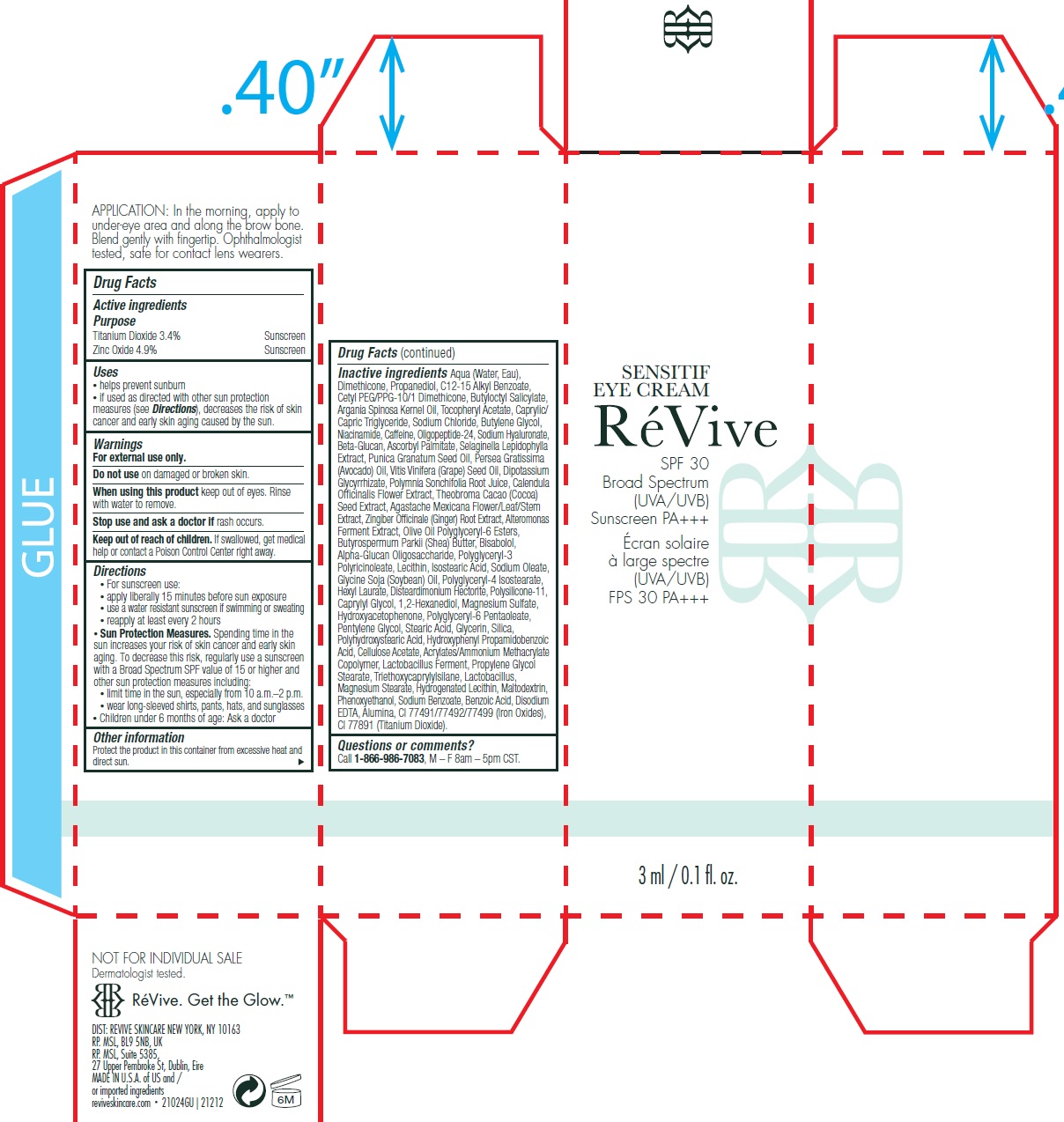
Label: SENSITIF EYE US SPF 30 BROAD SPECTRUM UVA UVB SUNSCREEN PA- titanium dioxide, zinc oxide cream
- NDC Code(s): 82691-144-00, 82691-144-01
- Packager: RV Skincare LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 6, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
• For sunscreen use: • apply liberally 15 minutes before sun exposure • use a water resistant sunscreen if swimming or sweating • reapply at least every 2 hours • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. – 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses • Children under 6 months of age: Ask a doctor
Sun Protection Measures. - Other information
-
Inactive ingredients
Aqua (Water, Eau), Dimethicone, Propanediol, C12-15 Alkyl Benzoate, Cetyl PEG/PPG-10/1 Dimethicone, Butyloctyl Salicylate, Argania Spinosa Kernel Oil, Tocopheryl Acetate, Caprylic/Capric Triglyceride, Sodium Chloride, Butylene Glycol, Niacinamide, Caffeine, Oligopeptide-24, Sodium Hyaluronate, Beta-Glucan, Ascorbyl Palmitate, Selaginella Lepidophylla Extract, Punica Granatum Seed Oil, Persea Gratissima (Avocado) Oil, Vitis Vinifera (Grape) Seed Oil, Dipotassium Glycyrrhizate, Polymnia Sonchifolia Root Juice, Calendula Officinalis Flower Extract, Theobroma Cacao (Cocoa) Seed Extract, Agastache Mexicana Flower/Leaf/Stem Extract, Zingiber Officinale (Ginger) Root Extract, Alteromonas Ferment Extract, Olive Oil Polyglyceryl-6 Esters, Butyrospermum Parkii (Shea) Butter, Bisabolol, Alpha-Glucan Oligosaccharide, Polyglyceryl-3 Polyricinoleate, Lecithin, Isostearic Acid, Sodium Oleate, Glycine Soja (Soybean) Oil, Polyglyceryl-4 Isostearate, Hexyl Laurate, Disteardimonium Hectorite, Polysilicone-11, Caprylyl Glycol, 1,2-Hexanediol, Magnesium Sulfate, Hydroxyacetophenone, Polyglyceryl-6 Pentaoleate, Pentylene Glycol, Stearic Acid, Glycerin, Silica, Polyhydroxystearic Acid, Hydroxyphenyl Propamidobenzoic Acid, Cellulose Acetate, Acrylates/Ammonium Methacrylate Copolymer, Lactobacillus Ferment, Propylene Glycol Stearate, Triethoxycaprylylsilane, Lactobacillus, Magnesium Stearate, Hydrogenated Lecithin, Maltodextrin, Phenoxyethanol, Sodium Benzoate, Benzoic Acid, Disodium EDTA, Alumina, CI 77491/77492/77499 (Iron Oxides), CI 77891 (Titanium Dioxide).
- Questions or comments?
- Package Labeling:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SENSITIF EYE US SPF 30 BROAD SPECTRUM UVA UVB SUNSCREEN PA
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82691-144 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 34 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 49 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) PROPANEDIOL (UNII: 5965N8W85T) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ARGAN OIL (UNII: 4V59G5UW9X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SODIUM CHLORIDE (UNII: 451W47IQ8X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) NIACINAMIDE (UNII: 25X51I8RD4) CAFFEINE (UNII: 3G6A5W338E) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ASCORBYL PALMITATE (UNII: QN83US2B0N) POMEGRANATE SEED OIL (UNII: 0UI45XV0T6) AVOCADO OIL (UNII: 6VNO72PFC1) GRAPE SEED OIL (UNII: 930MLC8XGG) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) COCOA (UNII: D9108TZ9KG) GINGER (UNII: C5529G5JPQ) OLIVE OIL POLYGLYCERYL-6 ESTERS (UNII: 4KDO9AFM9I) SHEA BUTTER (UNII: K49155WL9Y) LEVOMENOL (UNII: 24WE03BX2T) ISOSTEARIC ACID (UNII: X33R8U0062) SODIUM OLEATE (UNII: 399SL044HN) SOYBEAN OIL (UNII: 241ATL177A) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) PENTYLENE GLYCOL (UNII: 50C1307PZG) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPHENYL PROPAMIDOBENZOIC ACID (UNII: 25KRT26H77) CELLULOSE ACETATE (UNII: 3J2P07GVB6) PROPYLENE GLYCOL MONOSTEARATE (UNII: MZM1I680W0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM BENZOATE (UNII: OJ245FE5EU) BENZOIC ACID (UNII: 8SKN0B0MIM) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ALUMINUM OXIDE (UNII: LMI26O6933) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82691-144-00 1 in 1 CARTON 01/09/2021 1 15 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:82691-144-01 1 in 1 CARTON 01/09/2021 2 3 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/09/2021 Labeler - RV Skincare LLC (080986653)