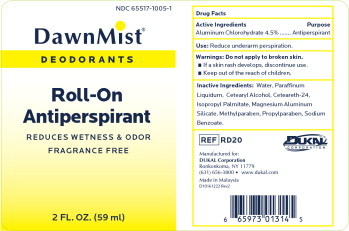
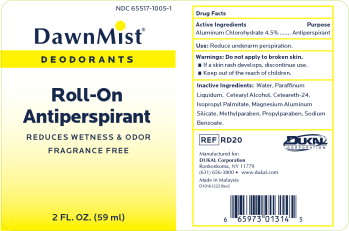
Label: DAWNMIST ANTIPERSPIRANT DEODORANT- aluminum chlorohydrate liquid
- NDC Code(s): 65517-1005-1
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 13, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Warnings
- Do not use
- Discontinue use if
- Ask a doctor before use
- Keep out of reach of children
- Directions
- Inactive Ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
DAWNMIST ANTIPERSPIRANT DEODORANT
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-1005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MINERAL OIL (UNII: T5L8T28FGP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-1005-1 59 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 01/10/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 01/10/2013 Labeler - Dukal LLC (791014871)