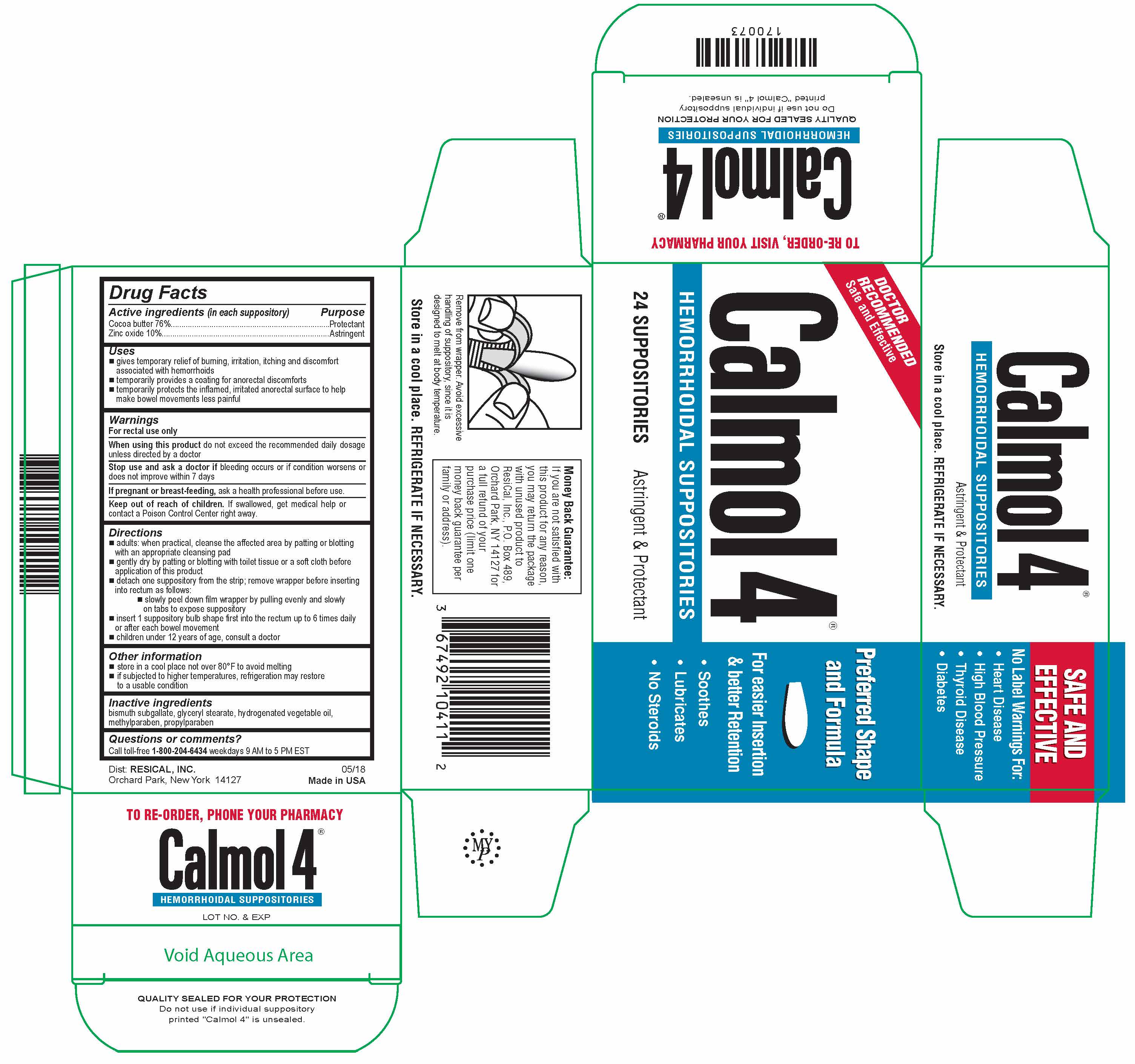
Label: CALMOL 4- zinc oxide and cocoa butter suppository
- NDC Code(s): 67492-104-11, 67492-104-12, 67492-104-13
- Packager: Resical Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 12, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (in each suppository)
- Purpose
- Uses
- Warnings
-
Directions
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing pad
- gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product
- detach one suppository from the strip; remove wrapper before inserting into rectum as follows:
- slowly peel down film wrapper by pulling evenly and slowly on tabs to expose suppository
- insert 1 suppository bulb shape first into the rectum up to 6 times daily or after each bowl movement
- children under 12 years of age, consult a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- Calmol 4 Suppository 24 count
-
INGREDIENTS AND APPEARANCE
CALMOL 4
zinc oxide and cocoa butter suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67492-104 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 270 mg COCOA BUTTER (UNII: 512OYT1CRR) (COCOA BUTTER - UNII:512OYT1CRR) COCOA BUTTER 2052 mg Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) FAT, HARD (UNII: 8334LX7S21) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) BISMUTH SUBGALLATE (UNII: YIW503MI7V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67492-104-11 4 in 1 CARTON 05/13/2006 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:67492-104-12 2 in 1 CARTON 05/13/2006 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:67492-104-13 1 in 1 CARTON 05/13/2006 3 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 05/13/2006 Labeler - Resical Inc. (927081369) Registrant - DSC Laboratories Inc. (097807374) Establishment Name Address ID/FEI Business Operations DSC Laboratories Inc. 097807374 manufacture(67492-104)