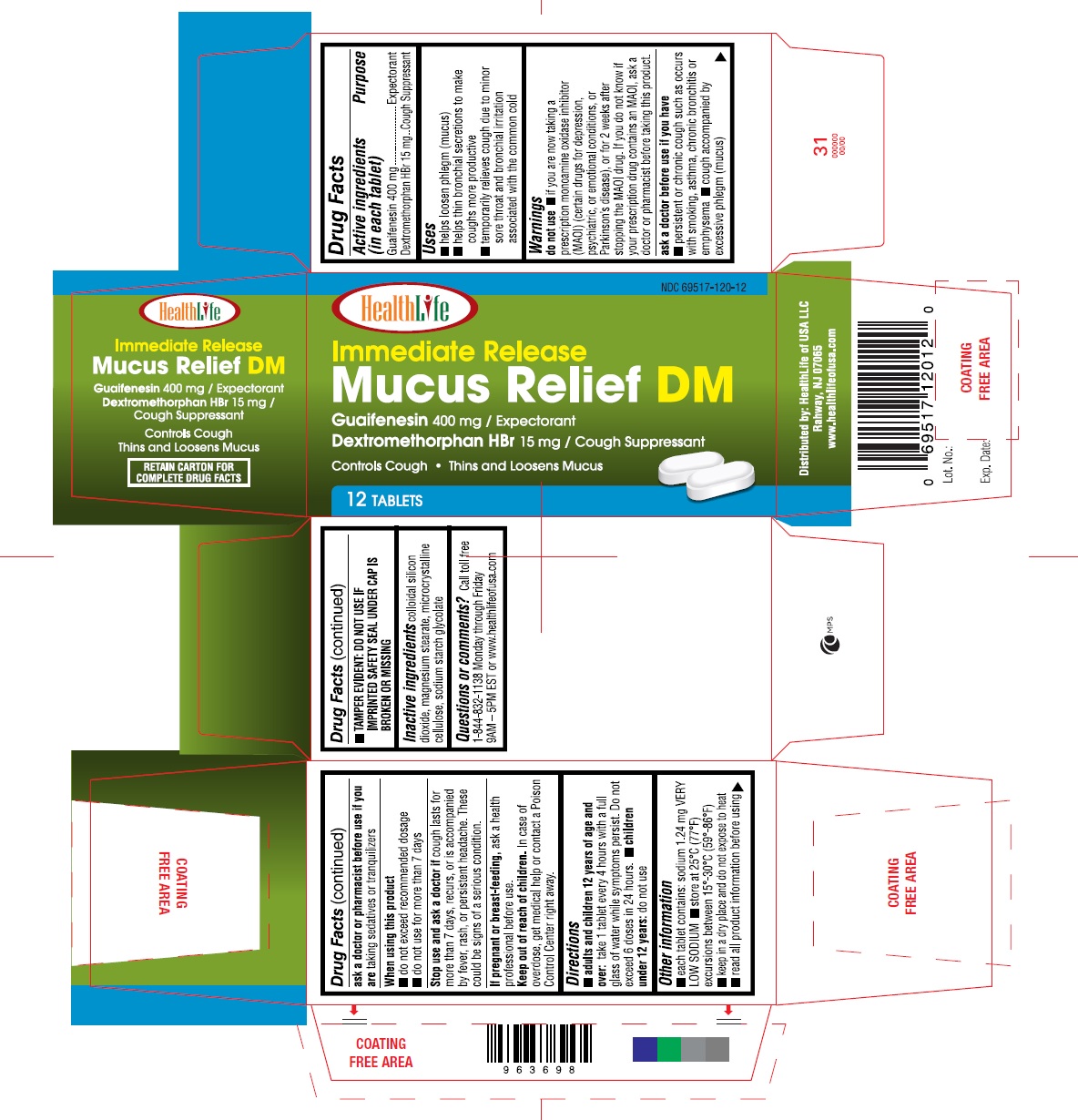
Label: MUCUS RELIEF DM- guaifenesin, dextromethorphan hbr tablets tablet
- NDC Code(s): 69517-120-12
- Packager: Healthlife of USA LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
-
Warnings
Do not use:
- If you are now taking a prescriprion monoamine oxidase inhibitor (MAOI) (Certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children
- Directions
- Other Information
- Inactive ingredients
- Questions or comments?
- Immediate Release Mucus Relief DM Guaifenesin 400mg / Expectorant Dextromethorphan HBr 15 mg/ Cough Suppressant Controls Cough Thins and Loosens Mucus 12 Tablets Distributed by: Healthlife of USA LLC Rahway NJ 07065 www.healthlifeofusa.com
-
INGREDIENTS AND APPEARANCE
MUCUS RELIEF DM
guaifenesin, dextromethorphan hbr tablets tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69517-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) Product Characteristics Color white Score no score Shape OVAL Size 22mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69517-120-12 1 in 1 CARTON 05/31/2017 1 12 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/31/2017 Labeler - Healthlife of USA LLC (079656178) Establishment Name Address ID/FEI Business Operations Elysium Pharmaceuticals Limited 915664486 manufacture(69517-120) , pack(69517-120)