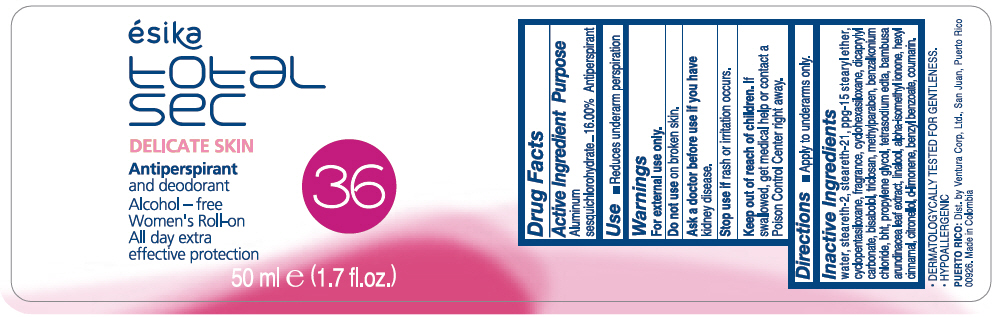
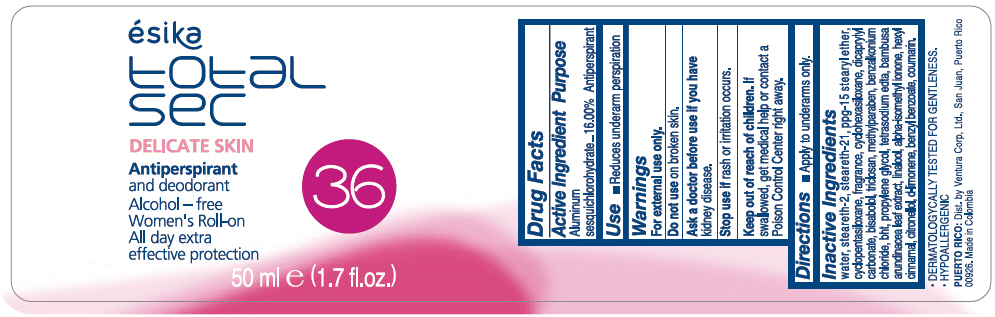
Label: ESIKA TOTAL SEC DELICATE SKIN ANTIPERSPIRANT AND DEODORANT- aluminum sesquichlorohydrate emulsion
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-965-01 - Packager: Ventura Corporation LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 16, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
WATER, STEARETH-2, STEARETH-21, PPG-15 STEARYL ETHER, CYCLOPENTASILOXANE, FRAGRANCE, CYCLOHEXASILOXANE, DICAPRYLYL CARBONATE, BISABOLOL, TRICLOSAN, METHYLPARABEN, BENZALKONIUM CHLORIDE, BHT, PROPYLENE GLYCOL, TETRASODIUM EDTA, BAMBUSA ARUNDINACEA LEAF EXTRACT, LINALOOL, ALPHA-ISOMETHYL IONONE, HEXYL CINNAMAL, CITRONELLOL, d-LIMONENE, BENZYL BENZOATE, COUMARIN.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
ESIKA TOTAL SEC DELICATE SKIN ANTIPERSPIRANT AND DEODORANT
aluminum sesquichlorohydrate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-965 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 0.16 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) PPG-15 STEARYL ETHER (UNII: 1II18XLS1L) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) LEVOMENOL (UNII: 24WE03BX2T) TRICLOSAN (UNII: 4NM5039Y5X) METHYLPARABEN (UNII: A2I8C7HI9T) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE SODIUM (UNII: MP1J8420LU) BAMBUSA BAMBOS LEAF (UNII: HW86D1FGSS) LINALOOL, (+/-)- (UNII: D81QY6I88E) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LIMONENE, (+)- (UNII: GFD7C86Q1W) BENZYL BENZOATE (UNII: N863NB338G) COUMARIN (UNII: A4VZ22K1WT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-965-01 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/12/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part350 07/12/2016 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-965)