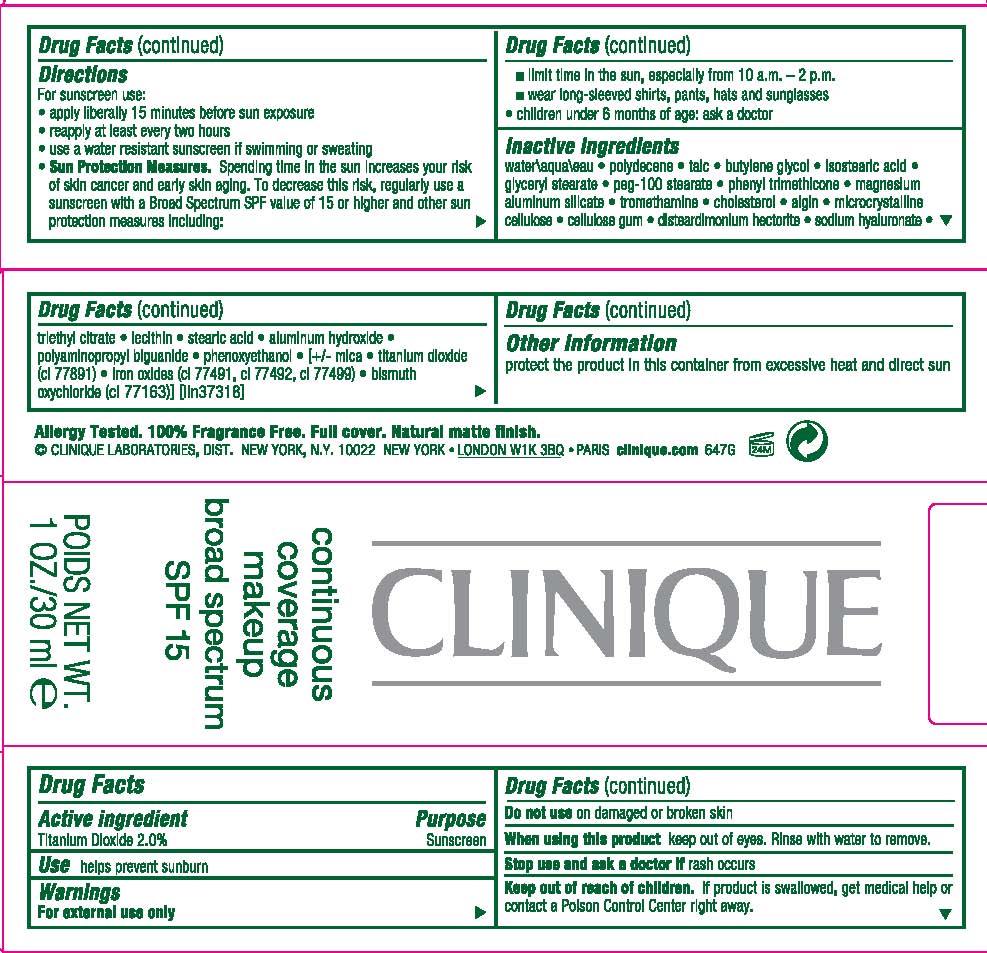
Label: CONTINUOUS COVERAGE MAKEUP SPF 15- titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 49527-010-01, 49527-010-02 - Packager: CLINIQUE LABORATORIES INC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 19, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- PURPOSE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
DIRECTIONS
- FOR SUNSCREEN USE:
- APPLY LIBERALLY 15 MINUTES BEFORE SUN EXPOSURE
- REAPPLY AT LEAST EVERY 2 HOURS
- USE A WATER RESISTANT SUNSCREEN IF SWIMMING OR SWEATING
- SUN PROTECTION MEASURES. SPENDING TIME IN THE SUN INCREASES YOUR RISK OF SKIN CANCER AND EARLY SKIN AGING. TO DECREASE THIS RISK, REGULARLY USE A SUNSCREEN WITH A BROAD SPECTRUM SPF VALUE OF 15 OR HIGHER AND OTHER SUN PROTECTION MEASURES INCLUDING:
-
- LIMIT TIME IN THE SUN, ESPECIALLY FROM 10 AM TO 2 PM
-
- WEAR LING-SLEEVED SHIRTS, PANTS AND SUNGLASSES
-
- CHILDREN UNDER 6 MONTHS OF AGE: ASK A DOCTOR
- CHILDREN UNDER 6 MONTHS OF AGE: ASK A DOCTOR
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS: water\aqua\eau [] polydecene [] talc [] butylene glycol [] isostearic acid [] glyceryl stearate [] peg-100 stearate [] phenyl trimethicone [] magnesium aluminum silicate [] tromethamine [] cholesterol [] algin [] microcrystalline cellulose [] cellulose gum [] disteardimonium hectorite [] sodium hyaluronate [] triethyl citrate [] lecithin [] stearic acid [] aluminum hydroxide [] polyaminopropyl biguanide [] phenoxyethanol [] [+/- mica [] titanium dioxide (ci 77891) [] iron oxides (ci 77491, ci 77492, ci 77499) [] bismuth oxychloride (ci 77163)
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CONTINUOUS COVERAGE MAKEUP SPF 15
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.0 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TALC (UNII: 7SEV7J4R1U) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ISOSTEARIC ACID (UNII: X33R8U0062) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) TROMETHAMINE (UNII: 023C2WHX2V) CHOLESTEROL (UNII: 97C5T2UQ7J) SODIUM ALGINATE (UNII: C269C4G2ZQ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYAMINOPROPYL BIGUANIDE (UNII: 322U039GMF) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) IRON (UNII: E1UOL152H7) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-010-01 1 in 1 CARTON 1 NDC:49527-010-02 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/01/1992 Labeler - CLINIQUE LABORATORIES INC (173047747) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 205952385 manufacture Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 manufacture Establishment Name Address ID/FEI Business Operations Len-Ron Manufacturing Division of Aramis Inc. 809771152 manufacture Establishment Name Address ID/FEI Business Operations Aramis Inc. 042918826 manufacture Establishment Name Address ID/FEI Business Operations Northtec Bristol 949264774 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Northtec Keystone 618107429 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Pennsylvania Distribution Center 2 828534516 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd. 255175580 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd 253616536 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Distribution Center 208579636 repack, relabel Establishment Name Address ID/FEI Business Operations Estee Lauder Kabushiki Kaisha 712808195 relabel, repack Establishment Name Address ID/FEI Business Operations Whitman Laboratories Ltd. 216866277 manufacture Establishment Name Address ID/FEI Business Operations Aveda Corporation 071352058 manufacture