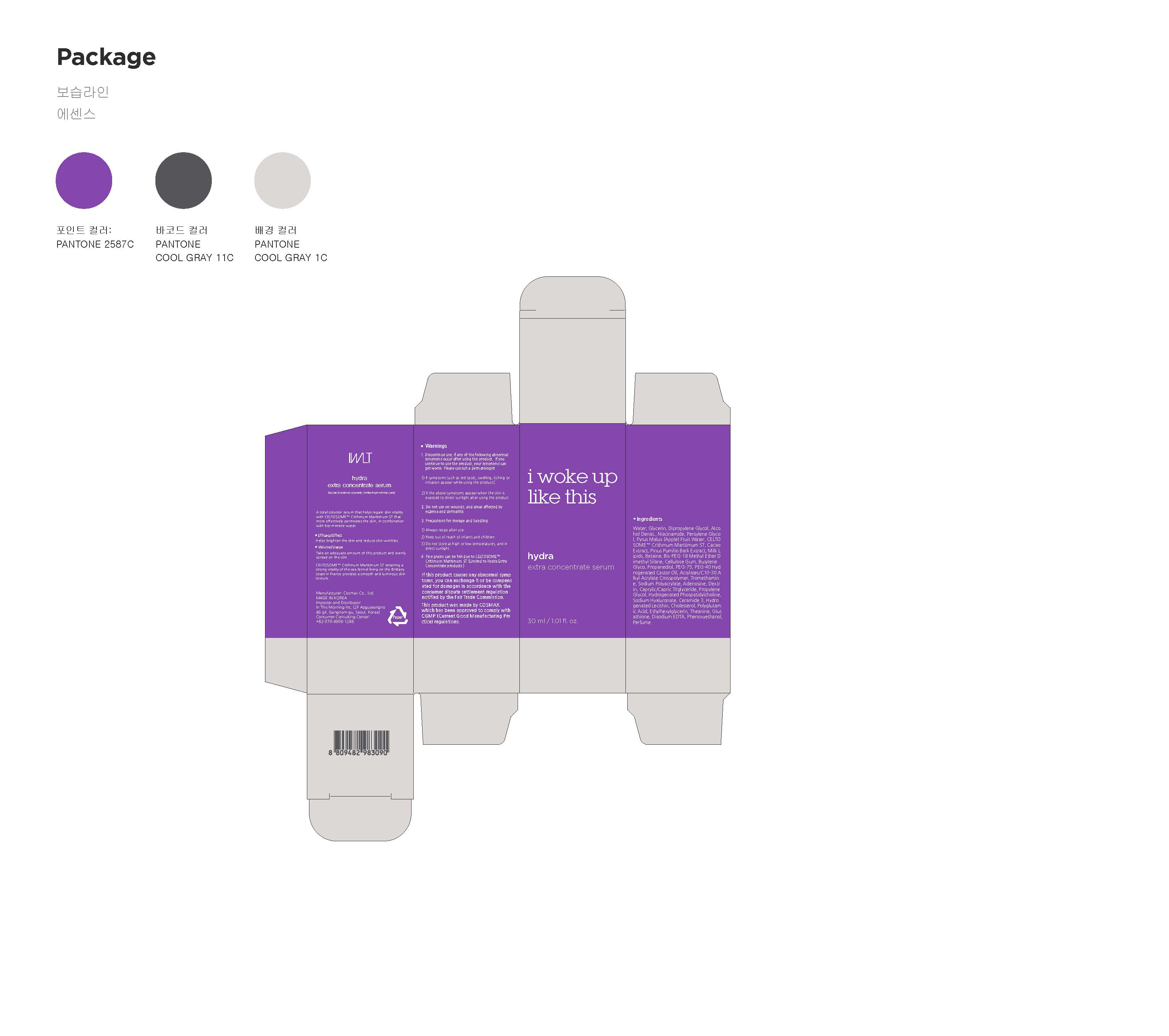
Label: HYDRA EXTRA CONCENTRATESERUM- niacinamide, adenosine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 71217-0004-1 - Packager: In This Morning
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 13, 2017
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
1. Discontinue use, if any of the following abnormal symptoms
occur after using the product. If you continue to use the
product, your symptoms can get worse. Please consult a
dermatologist.
1) If symptoms such as red spots, swelling, itching or irritation appear while
using the product
2) If the above symptoms appear when the skin is exposed to direct sunlight
after using the product
2. Do not use on wounds, and areas affected by eczema and
dermatitis
3. Precautions for storage and handling
1) Always recap after use.
2) Keep out of reach of infants and children.
3) Do not store at high or low temperatures, and in direct sunlight.
4. Avoid contact with the eyes.
- If you are very sensitive to a sticking plaster or a wet compress, use with
caution or discontinue use
- Limited to Whitening Concentrate Treatment Mask products.
- Fine grains can be felt due to CELTOSOME™ Crithmum Maritimum ST (Limited
to Hydra Extra Concentrate products.)
5. The SPF of this product was measured according to the
method of Cosmmedics Europe and its PA according to the
method of the Japan Cosmetic Industry Association (JCIA).
(Limited to Protective Base Sunscreen products for dry skin)
6. The SPF of this product was measured according to the
International SPF Test Method and its PA according to the
method of the Japan Cosmetic Industry Association (JCIA).
(Limited to Protective Base Sunscreen products for oily skin)
7. The SPF and PA of this product were measured according
to the methods proposed by the Regulations of Functional
Cosmetic Evaluation. (Limited to BB cream.)
8. Do not use on children under the age of 3 because this
product contains salicylic acids and salts.
(Limited to Purifying Sebum Relief Spot products.)
If this product causes any abnormal symptoms,
you can exchange it or be compensated for damages in
accordance with the consumer dispute settlement regulation
notified by the Fair Trade Commission.
This product was made by COSMAX
which has been approved to comply with
CGMP (Current Good Manufacturing Practice) regulations. - DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYDRA EXTRA CONCENTRATESERUM
niacinamide, adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71217-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 100 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71217-0004-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 02/08/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/08/2017 Labeler - In This Morning (694519423) Registrant - In This Morning (694519423) Establishment Name Address ID/FEI Business Operations In This Morning 694519423 label(71217-0004) , pack(71217-0004) , manufacture(71217-0004)