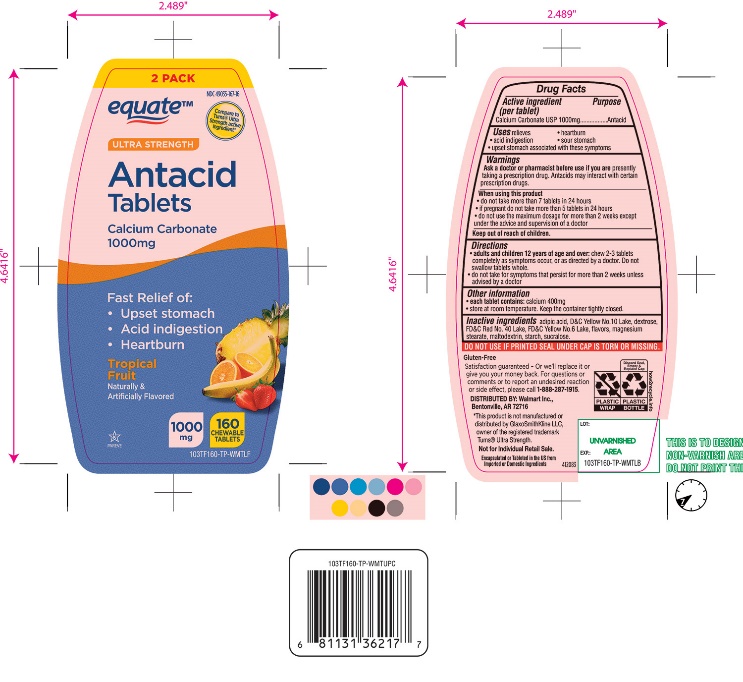
Label: ULTRA STRENGTH ANTACID- calcium carbonate tablet, chewable
- NDC Code(s): 49035-167-16
- Packager: Wal-Mart Stores,Inc.,
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (per tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- •
- do not take more than 7 tablets in 24 hours
- •
- if pregnant do not take more than 5 tablets in 24 hours
- •
- do not use the maximum dosage for more than 2 weeks except under the advice and supervision of a doctor.
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
-
Package/Label Principal Display Panel
NDC 49035-167-16
*Compare to the active ingredient in Ultra Strength Tums®
ULTRA STRENGTH
Antacid Tablets
Calcium Carbonate 1000 mg
Fast Relief of Upset Stomach, Heartburn and Acid Indigestion.
Tropical Fruit
Naturally and Artificially Flavored
160 CHEWABLE TABLETS
GLUTEN- FREE
Satisfaction guaranteed- Or we will replace it or give you your money back. For questions or to report an undesired reaction or side effect, please call 1-888-287-1915.
Encapsulated or Tableted in the US from Imported or Domestic Ingredients.
Distributed by: Wal-Mart Stores, Inc.,
Bentonville, AR 72716
*This product is not manufactured or distributed by GlaxoSmithKline LLC, owner of the registered trademark, Ultra Strength Tums®.
Not for individual Retail Sale.
Encapsulated or Tableted in the US from Imported or Domestic Ingredients
-
INGREDIENTS AND APPEARANCE
ULTRA STRENGTH ANTACID
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-167 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 1000 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) STARCH, CORN (UNII: O8232NY3SJ) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color YELLOW, PINK, ORANGE, WHITE Score no score Shape ROUND Size 19mm Flavor FRUIT (tropical fruit flavor) Imprint Code RP103 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-167-16 160 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/18/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 11/18/2019 Labeler - Wal-Mart Stores,Inc., (051957769)