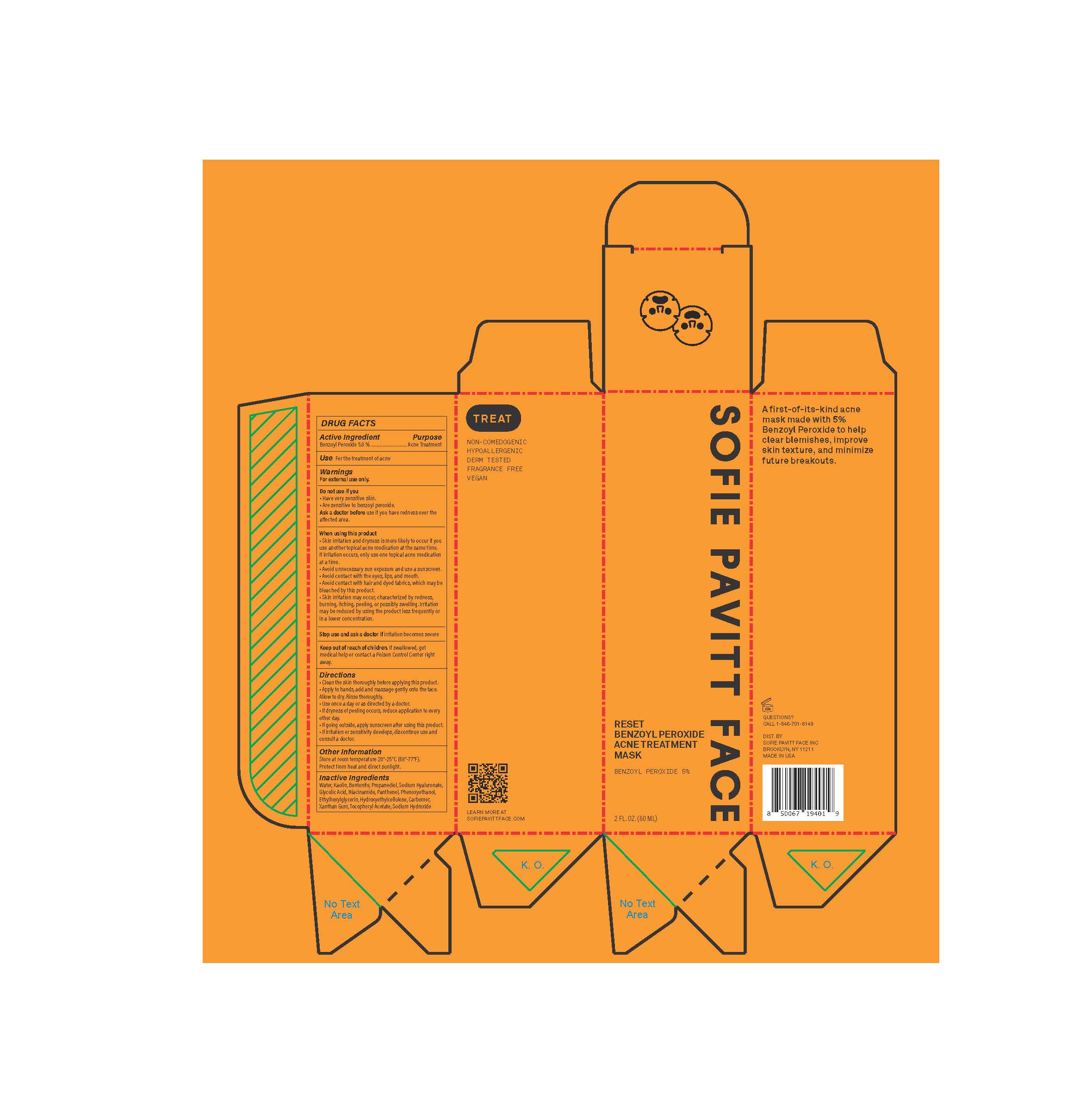
Label: SOFIE PAVITT FACE RESET BENZOYL PEROXIDE ACNE TREATMENT MASK- benzoyl peroxide paste
- NDC Code(s): 52261-7301-1
- Packager: Cosco International, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient/Purpose
- Use
-
Warnings
For external use only
Do not use if you
- have very sensitive skin
- are sensitive to benzoyl peroxide
Ask doctor before use
if you have redness over the affected area
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Avoid unnecessary sun exposure and use a sunscreen
- Avoid contact with the eyes, lips, and mouth
- Avoid contact with hair and dyed fabrics, which may be bleached by this product
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- Clean the skin throughly before applying this product
- Apply to hands, add and massage gently onto the face. Allow to dry. Rinse thoroughly.
- Use once a day or as directed by a doctor.
- If dryness or peeling occurs, reduce application to once every other day.
- If going outside, apply sunscreen after using this product.
- If irritation or sensitivity develops, discontinue use and consult a doctor.
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SOFIE PAVITT FACE RESET BENZOYL PEROXIDE ACNE TREATMENT MASK
benzoyl peroxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52261-7301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzoyl Peroxide (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) Benzoyl Peroxide 0.05 kg in 1 kg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) KAOLIN (UNII: 24H4NWX5CO) BENTONITE (UNII: A3N5ZCN45C) PROPANEDIOL (UNII: 5965N8W85T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCOLIC ACID (UNII: 0WT12SX38S) NIACINAMIDE (UNII: 25X51I8RD4) PANTHENOL (UNII: WV9CM0O67Z) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER (UNII: 0A5MM307FC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52261-7301-1 1 in 1 CARTON 11/11/2024 1 0.0618 kg in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 11/11/2024 Labeler - Cosco International, Inc. (016433141) Registrant - Cosco International, Inc. (016433141) Establishment Name Address ID/FEI Business Operations Cosco International, Inc. 016433141 manufacture(52261-7301) , label(52261-7301) , pack(52261-7301)