Label: PVP-I POUCH FOIL-FOIL- povidone-iodine solution
- NDC Code(s): 65517-0035-1, 65517-0035-2
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 28, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient:
- Purpose:
- Use:
-
Warnings:
For external use only.
Do not apply to persons allergic to iodine. Do not use in the eyes.
- Ask a doctor before use if injuries are deep wounds, puncture wounds, serious burns.
- Directions:
- Other Information:
- Inactive Ingredients:
-
Principal Display Panel - 3/4 oz Pouch Label
Dukal
Povidone-Iodine3/4 Fluid Ounce
Solution
Sterile
Antiseptic
Drug Facts
Active Ingredient Purpose
Povidone Iodine USP 10% Antiseptic
Use Patient preoperative skin reparation: Helps to reduce bacteria that potentially can cause skin infection.
Sterile unless package is damaged or opened.
0.75 fl oz (22.5 ml) - 2 - NDC 6551-0035-1 - REF 885

-
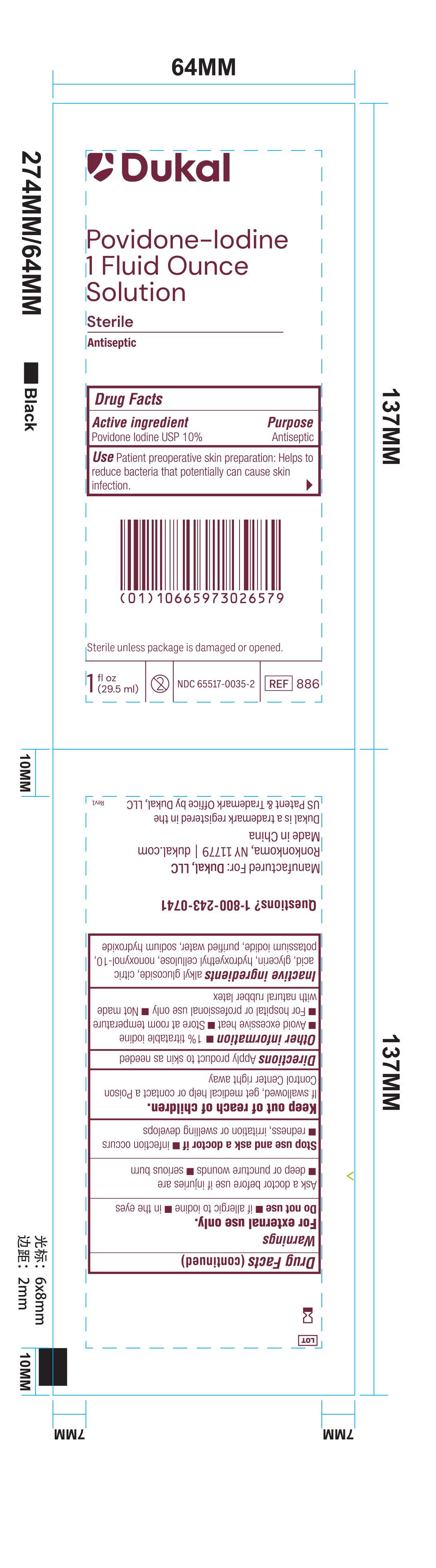
Principal Display Panel - 1 oz Pouch Label
Dukal
Povidone-Iodine1 Fluid Ounce
Solution
Sterile
Antiseptic
Drug Facts
Active Ingredient Purpose
Povidone Iodine USP 10% Antiseptic
Use Patient preoperative skin reparation: Helps to reduce bacteria that potentially can cause skin infection.
Sterile unless package is damaged or opened.
1 fl oz (29.5 ml) - 2 - NDC 6551-0035-2 - REF 886
-
INGREDIENTS AND APPEARANCE
PVP-I POUCH FOIL-FOIL
povidone-iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-0035 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) NONOXYNOL-10 (UNII: K7O76887AP) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) POTASSIUM IODIDE (UNII: 1C4QK22F9J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-0035-1 500 in 1 CASE 01/31/2018 1 22 mL in 1 POUCH; Type 0: Not a Combination Product 2 NDC:65517-0035-2 250 in 1 CASE 11/09/2018 2 29.57 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/31/2018 Labeler - Dukal LLC (791014871)


