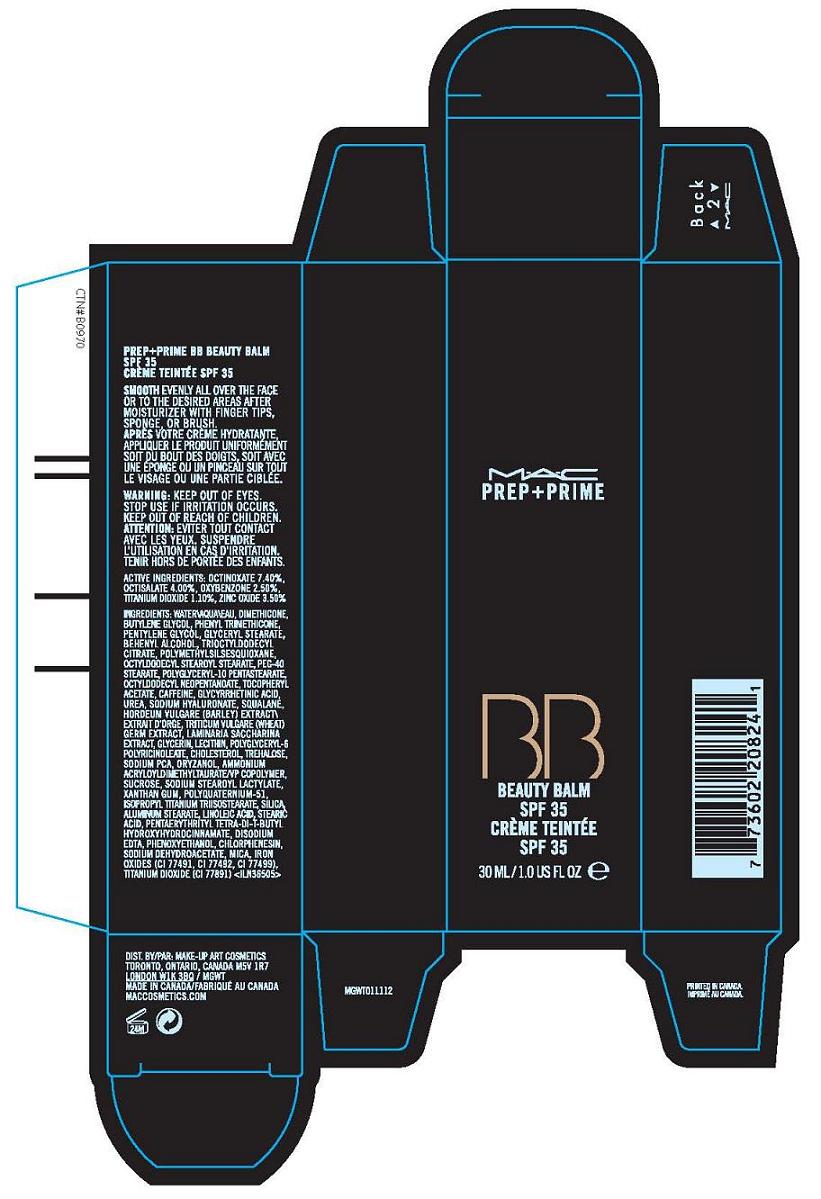
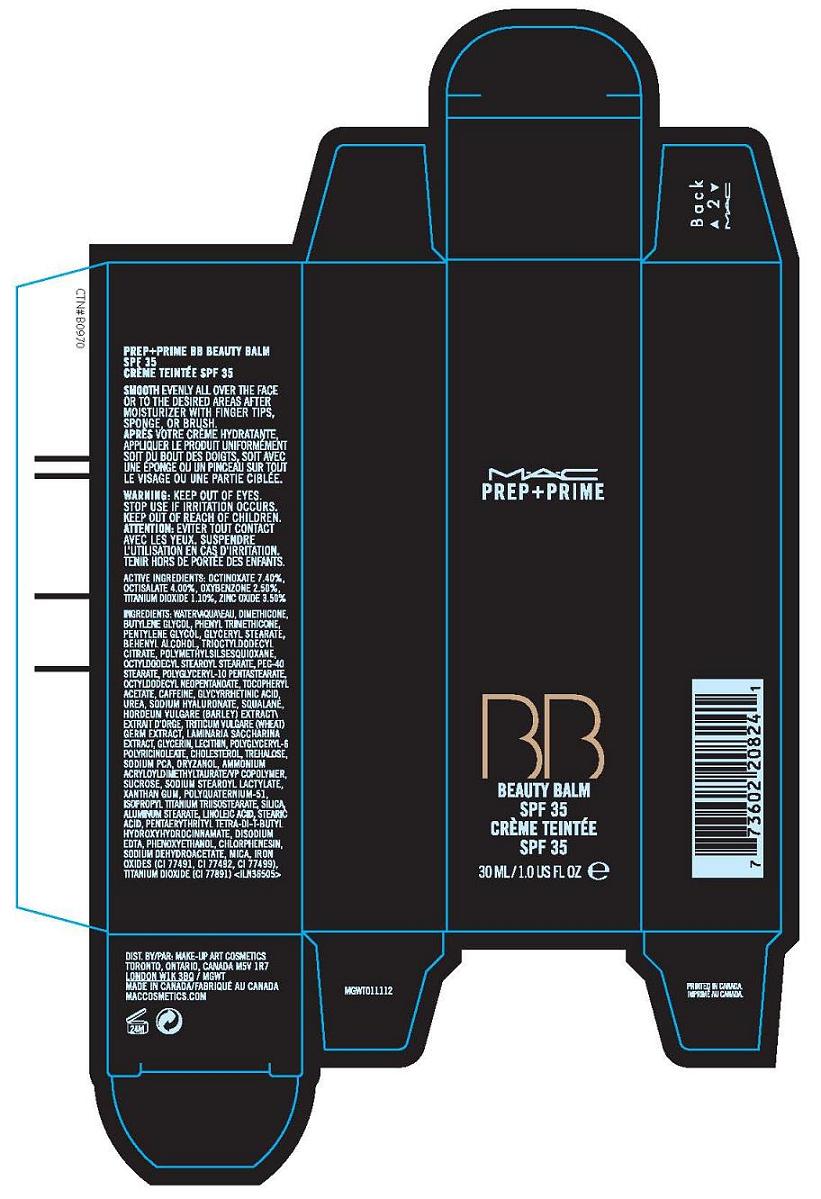
Label: PREP (PLUS) PRIME BEAUTY BALM SPF 35- octinoxate, octisalate, oxybenzone, titanium dioxide, zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 40046-0036-1, 40046-0036-2 - Packager: MAKE-UP ART COSMETICS
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 14, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WHEN USING
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREP (PLUS) PRIME BEAUTY BALM SPF 35
octinoxate, octisalate, oxybenzone, titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:40046-0036 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.4 mL in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4 mL in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 2.5 mL in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.1 mL in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 3.5 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:40046-0036-1 1 in 1 CARTON 1 NDC:40046-0036-2 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/01/2010 Labeler - MAKE-UP ART COSMETICS (010597206) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 205952385 manufacture Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 manufacture Establishment Name Address ID/FEI Business Operations Len-Ron Manufacturing Division of Aramis Inc. 809771152 manufacture Establishment Name Address ID/FEI Business Operations Aramis Inc. 042918826 manufacture Establishment Name Address ID/FEI Business Operations Northtec Bristol 949264774 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Northtec Keystone 618107429 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations PADC 1 110482184 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Pennsylvania Distribution Center 2 828534516 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd. 255175580 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd 253616536 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Distribution Center 208579636 repack, relabel Establishment Name Address ID/FEI Business Operations Estee Lauder Kabushiki Kaisha 712808195 relabel, repack Establishment Name Address ID/FEI Business Operations Whitman Laboratories Ltd. 216866277 manufacture