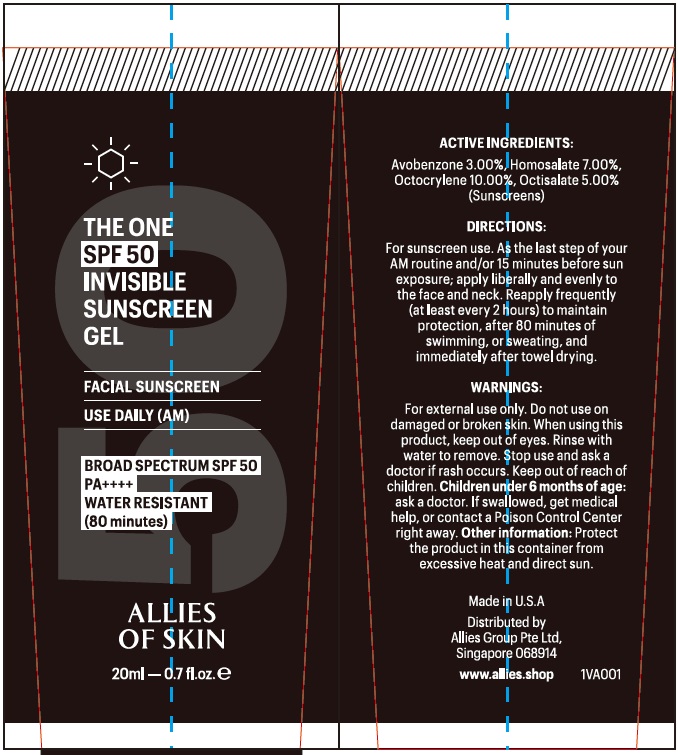
Label: ALLIES OF SKIN THE ONE SPF-50 INVISIBLE SUNSCREEN- avobenzone, homosalate, octocrylene, octisalate gel
- NDC Code(s): 83852-438-03, 83852-438-20, 83852-438-50
- Packager: ALLIES GROUP PTE. LTD.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 25, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses:
- Warnings:
-
Directions:
• Apply liberally 15 minutes before sun exposure and as needed. • children under 6 months of age: ask a doctor.Reapply:• after 80 minutes of swimming or sweating • immediately after towel drying • at least every 2 hours
• Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with broad spectrum SPF of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. – 2 p.m. • Wear long-sleeved shirts, pants, hats and sunglasses.
- Other Information:
-
Inactive Ingredients:
Isododecane, Dimethicone/bis-lsobutyl PPG-20 Crosspolymer, Butyloctyl Salicylate, Caprylic/Capric Triglyceride, Tridecyl Salicylate, Aloe Barbadensis Leaf Extract, Camellia Sinensis (Green Tea) Extract, Citrullus Lanatus (Watermelon) Extract, Daucus Carota Sativa (Carrot) Extract, Glycyrrhiza Glabra (Licorice) Extract, Helianthus Annuus (Sunflower) Oil, Punica Granatum (Pomegranate) Extract, Rosmarinus Officinalis (Rosemary) Extract, Rubus Idaeus (Raspberry) Oil, Niacinamide, Tetrahexyldecyl Ascorbate (Vitamin C), Tocopherol (Vitamin E)
- Package Labeling:50ml
- Package Labeling:3ml
- Package Labeling: 20ml
-
INGREDIENTS AND APPEARANCE
ALLIES OF SKIN THE ONE SPF-50 INVISIBLE SUNSCREEN
avobenzone, homosalate, octocrylene, octisalate gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83852-438 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 70 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TRIDECYL SALICYLATE (UNII: AZQ08K38Z1) ALOE VERA LEAF (UNII: ZY81Z83H0X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) WATERMELON (UNII: 231473QB6R) CARROT (UNII: L56Z1JK48B) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) PUNICA GRANATUM ROOT BARK (UNII: CLV24I3T1D) ROSEMARY (UNII: IJ67X351P9) NIACINAMIDE (UNII: 25X51I8RD4) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83852-438-50 1 in 1 BOX 12/11/2023 1 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:83852-438-03 3 mL in 1 PACKET; Type 0: Not a Combination Product 12/11/2023 3 NDC:83852-438-20 1 in 1 BOX 06/19/2024 3 20 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/11/2023 Labeler - ALLIES GROUP PTE. LTD. (595420087)