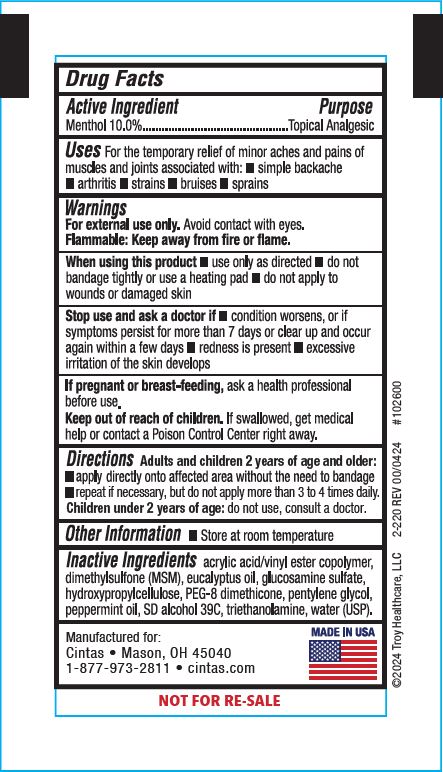
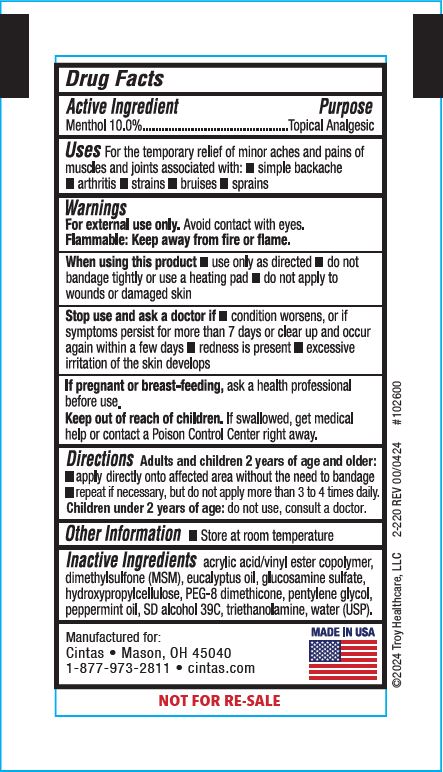
Label: XPECT STOPAIN CLINICAL- menthol gel
- NDC Code(s): 42961-256-01
- Packager: Cintas Corporation
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated June 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only. Avoid contact with eyes.
Flammable: Keep away from fire or flame.
When using this product
- use only as directed
- do not bandage tightly or use a heating pad
- do not apply to wounds or damaged skin
- Directions
- Other Information
- Inactive Ingredients
-
Principal Display Panel 42961-256-01
Stopain® Clinical
Maximum Performance Pain Relief Gel
Immediate, Penetrating Relief from:
- Arthritis
- Muscle Aches
- Joint & Back Pain
10% Menthol With MSM & Glucosamine
NET WT. (3g)
Xpect® First aid

Manufactured for: Cintas • Mason, OH 45040
1-877-973-2811 • cintas.com
MADE IN USA
NOT FOR RE-SALE
©2024 Troy Healthcare, LLC 2-220 REV 00/0424 #102600
-
INGREDIENTS AND APPEARANCE
XPECT STOPAIN CLINICAL
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42961-256 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 100 mg in 1 g Inactive Ingredients Ingredient Name Strength ACRYLIC ACID (UNII: J94PBK7X8S) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) EUCALYPTUS OIL (UNII: 2R04ONI662) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) PEG-8 DIMETHICONE (UNII: GIA7T764OD) PENTYLENE GLYCOL (UNII: 50C1307PZG) PEPPERMINT OIL (UNII: AV092KU4JH) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42961-256-01 3 g in 1 PACKET; Type 0: Not a Combination Product 06/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/14/2024 Labeler - Cintas Corporation (056481716) Establishment Name Address ID/FEI Business Operations Troy Manufacturing, Inc. 160075248 manufacture(42961-256)