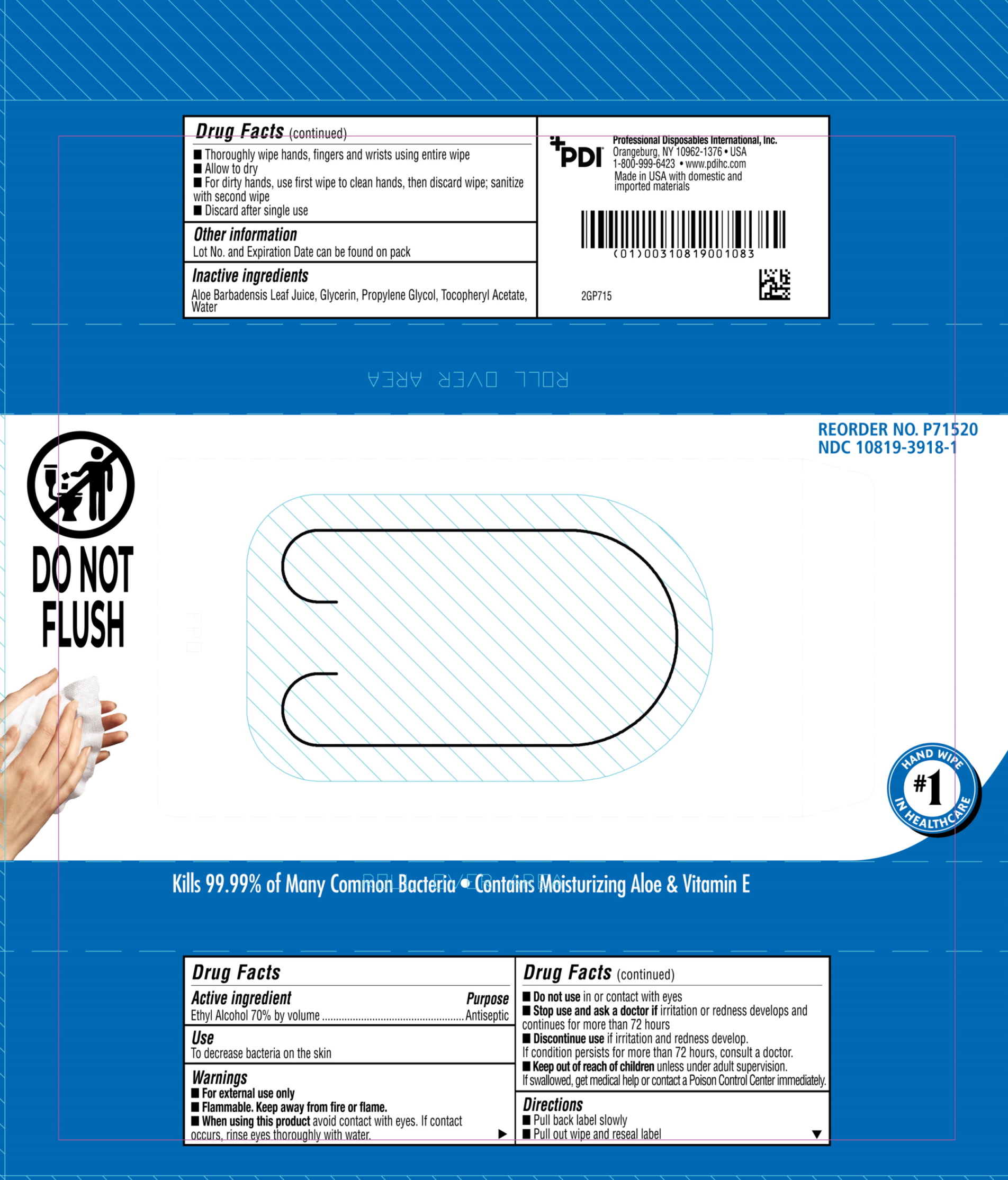
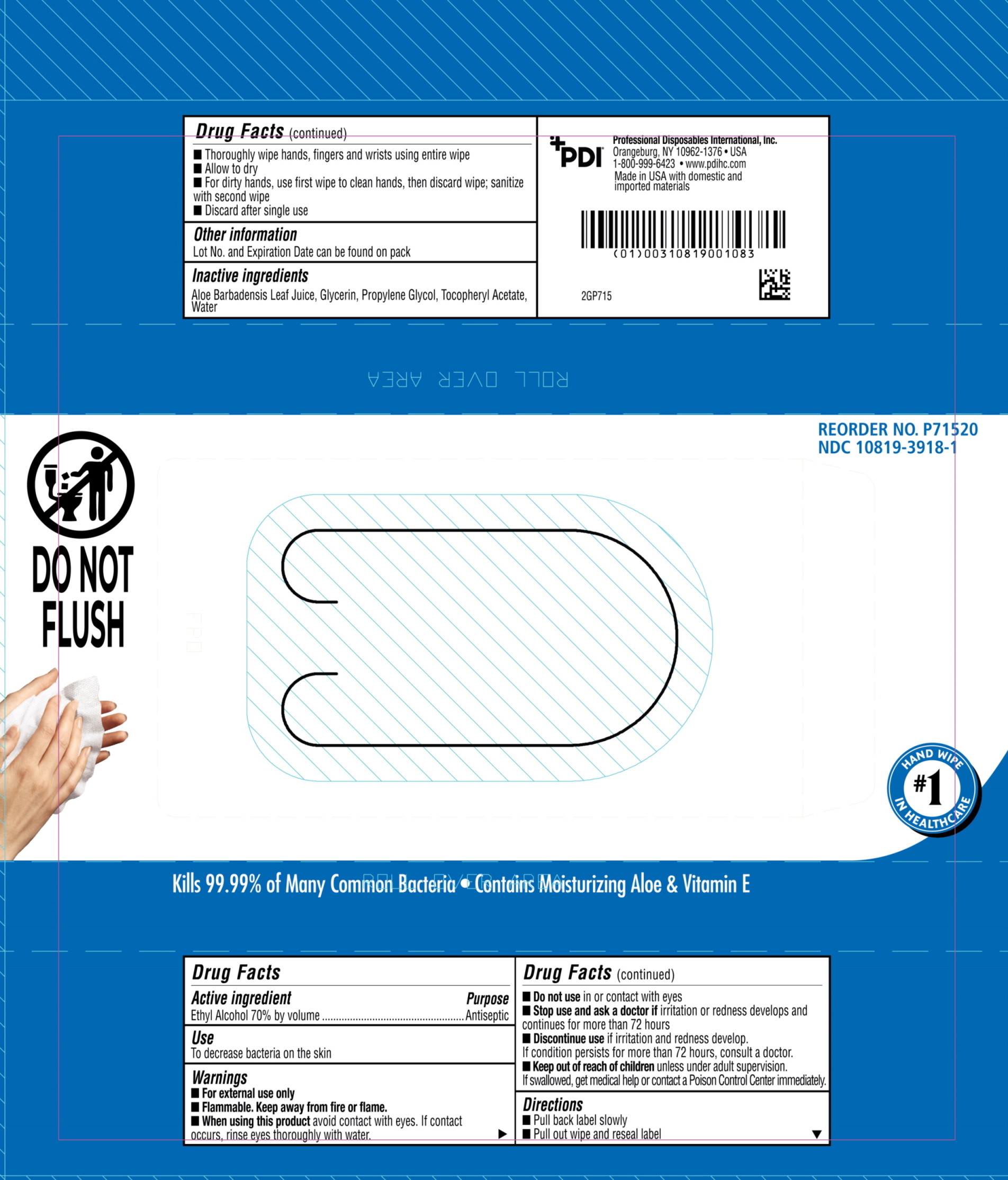
Label: PDI SANI HANDS- instant hand sanitizing wipes cloth
- NDC Code(s): 10819-3918-1
- Packager: Professional Disposables International Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 21, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep this out of reach of children
-
Directions
- Peel back label slowly.
- Pull out wipe and reseal label.
- Unfold and use.
- Wipe hands, fingers, interdigital areas and wrists thoroughly with towelette. Be sure to utilize the entire wipe surface. Allow to dry.
- If hands are visibly soiled or contaminated, use first wipe to clean hands, then discard wipe. Sanitize with a second wipe.
- Discard after single use.
- inactive ingredients
- Principal Display panel
-
INGREDIENTS AND APPEARANCE
PDI SANI HANDS
instant hand sanitizing wipes clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10819-3918 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10819-3918-1 20 in 1 CELLO PACK 04/01/2016 1 4.15 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/01/2016 Labeler - Professional Disposables International Inc (800777117) Registrant - Professional Disposables International Inc (800777117)