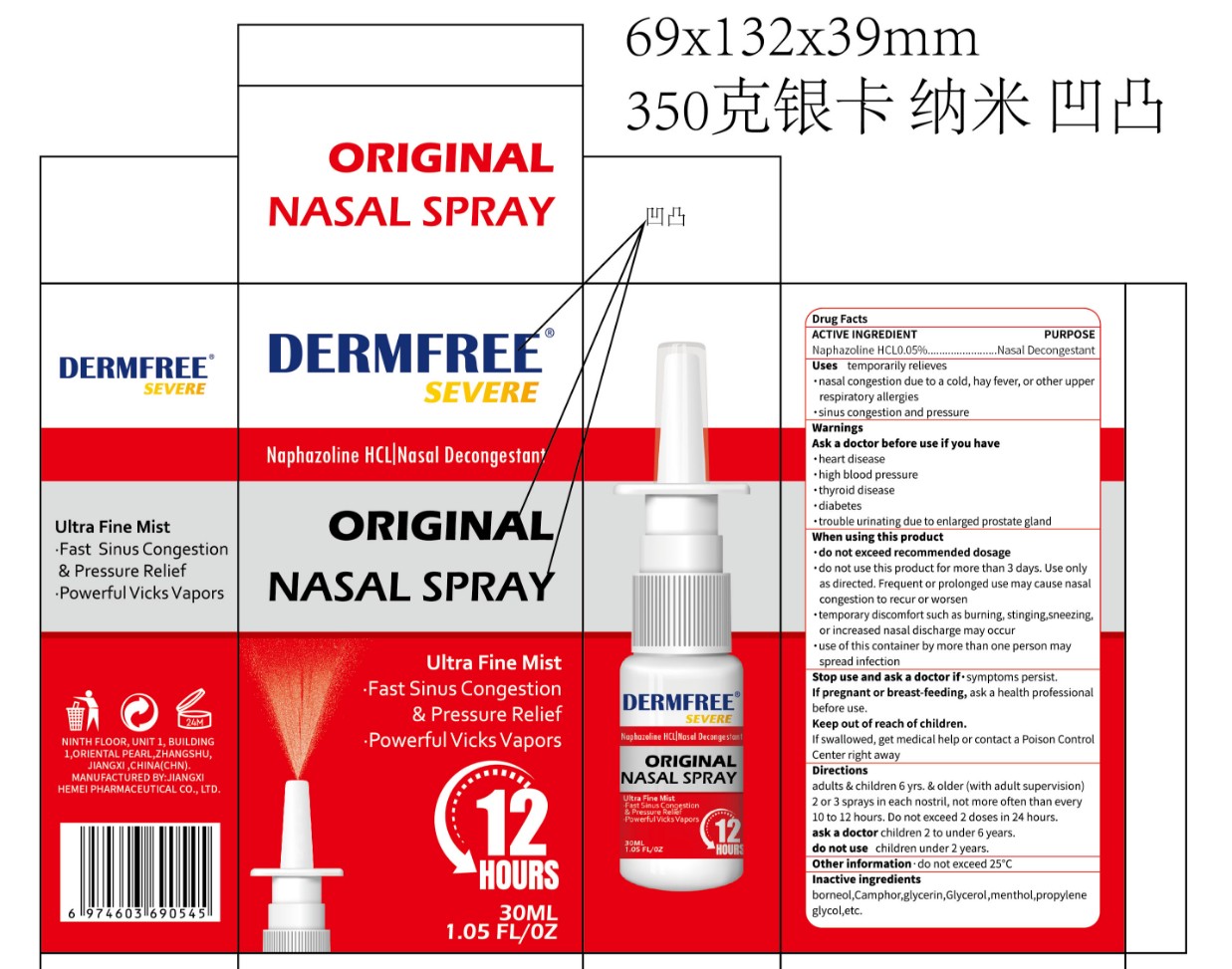
Label: DERMFREE ORIGINAL NASAL- naphazoline hcl 0.05%original nasal spray
- NDC Code(s): 84010-057-01
- Packager: Jiangxi Hemei Pharmaceutical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
-
When Using
·do not exceed recommended dosage
·do not use this product for more than 3 days. Use only as directed. Frequentor prolonged use may cause nasal congestion to recur or worsentemporary discomfort such as burning, stinging,sneezing, or increased nasaldischarge may occur
·temporary discomfort such as burning, stinging, sneezing, or increased nasal discharge may occur
·use of this container by more than one person may spread infection - Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMFREE ORIGINAL NASAL
naphazoline hcl 0.05%original nasal sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84010-057 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPHAZOLINE HCL (UNII: MZ1131787D) (NAPHAZOLINE - UNII:H231GF11BV) NAPHAZOLINE HCL 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL (UNII: L7T10EIP3A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BORNEOL (UNII: M89NIB437X) DIGLYCERIN (UNII: 3YC120743U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84010-057-01 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 12/19/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/19/2024 Labeler - Jiangxi Hemei Pharmaceutical Co., Ltd (724892056) Establishment Name Address ID/FEI Business Operations Jiangxi Hemei Pharmaceutical Co., Ltd 724892056 manufacture(84010-057)